
We Have Got You Covered – Major Data Readouts at ASCO 2021 Annual Meeting
- Home
- asco conference 2021
- asco 2021 annual meeting
ASCO 2021 Annual Meeting - Major Data Readouts
The ASCO 2021 annual meeting conference will be held from June 4 to June 8, 2021, and while many abstracts have been released on the website, some are still under wraps. Of them, Delveinsight has listed the major late-breaking presentations with insights on a few of them that are due to be presented at the plenary session.
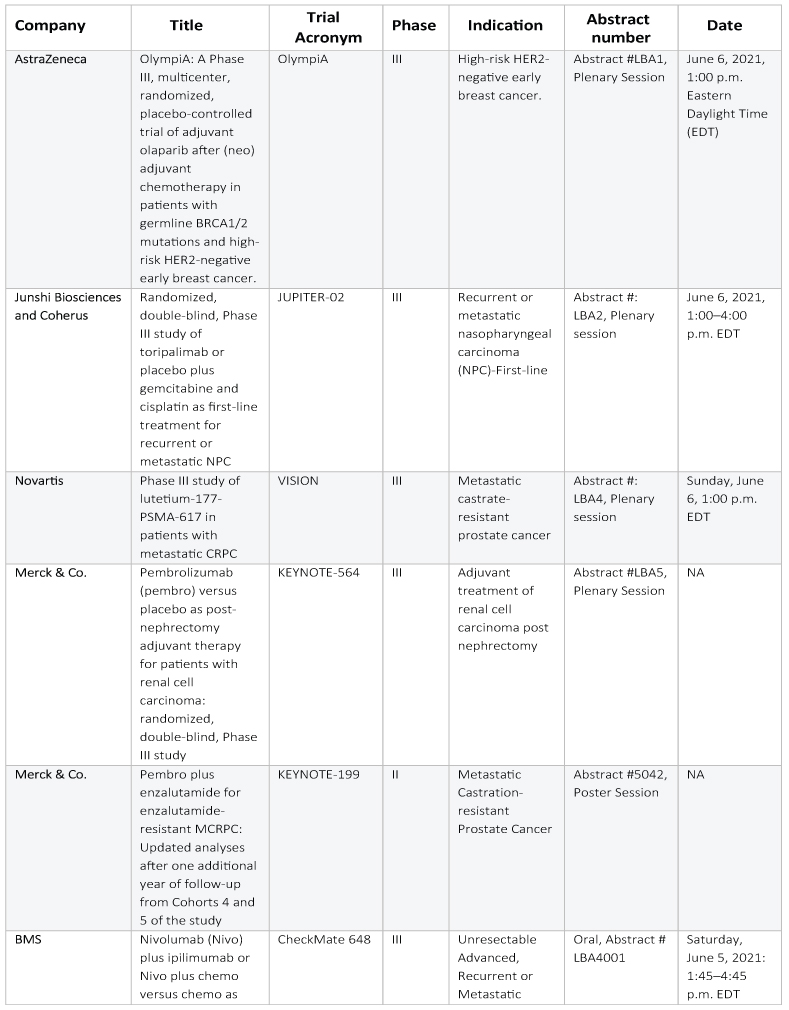
AstraZeneca’s Lynparza in Early Breast Cancer
The upcoming data readout from the Phase III OlympiA study can stir up a new commercial opportunity for AstraZeneca in the adjuvant setting, where Lynparza is targeting germline BRCA mutant (gBRCAm) high-risk HER2-negative early breast cancer patients who have completed definitive local treatment and neoadjuvant or adjuvant chemotherapy. The company has already announced plans to file global regulatory submissions for this PARP inhibitor as an adjuvant treatment for high-risk HER2-negative early breast cancer in the second half of 2021.
Although the drug is already a standard of care for gBRCAm advanced breast cancer patients, we are pretty excited for the upcoming label expansion once the data is released.
Shanghai Junshi’s Toripalimab in Nasopharyngeal Cancer
Shanghai Junshi Biosciences will be unveiling the first results from the JUPITER-02 trial of its anti-PD-1 antibody Toripalimab in front-line nasopharyngeal cancer. In September 2020, the company announced the positive interim analysis where it met its prespecified primary endpoint. The study results showed that toripalimab combined with gemcitabine/cisplatin as a first-line treatment for patients with recurrent or metastatic nasopharyngeal carcinoma (NPC) significantly extended the progression-free survival (PFS) than the current standard treatment of gemcitabine/cisplatin.
For a wider spread of the asset, the company announced a collaboration with Coherus BioSciences to bring the drug to the US. In addition, a rolling BLA submission has been initiated with the FDA to treat recurrent or metastatic nasopharyngeal carcinoma.
Novartis’s Lutetium 177Lu-PSMA-617 for Prostate Cancer
The first look of Novartis’ Phase III VISION study of its prostate-specific membrane antigen (PSMA)-directed radiopharmaceutical Lutetium 177Lu-PSMA-617 in PSMA-positive metastatic castration-resistant prostate cancer (mCPRC) patients. Interestingly, the trial met both primary endpoints of overall survival (OS) and radiographic PFS, helping to move closer to the ambition of becoming the targeted treatment for >80% of patients with advanced prostate cancer. If approved, this radioligand therapy could fulfill an unmet need where high PSMA expression is associated with higher Gleason grade and worse OS. Since regulatory filings based on these results are expected in the EU and US during the second half of 2021, we are eagerly waiting to watch out for the results.
Merck’s Keytruda for Renal Cell Cancer (RCC)
Merck’s blockbuster PD-1, Keytruda has already received priority review from FDA with its TKI partner, Lenvima, last month. Now we are eagerly waiting for results from Merck’s KEYNOTE-564, a Phase III trial evaluating the use of postoperative Keytruda (pembrolizumab) in locally advanced RCC. In April 2021, the company announced that the pivotal trial met its primary endpoint of disease-free survival (DFS) and demonstrated a statistically significant and clinically meaningful improvement in DFS compared with placebo. Since the earlier settings of the disease lack availability of targeted therapies, approval of pembrolizumab could fill a void in the adjuvant setting. Also, this asset could boost the upcoming revenue growth for the company, if approved, against its rival BMS’ Opdivo, which is also seeking to establish Opdivo as adjuvant therapy for locally advanced tumors in Phase III CheckMate 914 trial.
Bristol Myers Squibb’s Opdivo for Esophageal Cancer
BMS is set to present the first quantitative results from the Phase III CheckMate 648 trial in first-line esophageal squamous cell carcinoma (ESCC) with all high spirits. In April 2021, the company announced the positive topline results from the study, demonstrating a statistically significant and clinically meaningful benefit for the primary and secondary endpoints of OS.
The trial is similar to that of CheckMate 649, based on which Opdivo combined with chemotherapy was approved for advanced or metastatic gastric cancer (GC), gastroesophageal junction cancer (GEJC), and esophageal adenocarcinoma (EAC). However, Checkmate-648 differs in histology and has recruited patients with ESCC subtype of esophageal cancer.
Opdivo will encounter competition from Keytruda’s KEYNOTE-590 regimen, which has recruited both EACs and ESCCs. Importantly, the US FDA has already accepted and granted priority review for a new supplemental Biologics License Application (sBLA) for pembrolizumab.
The rest of the key abstracts have been summarized in the table below:
Executive Summary
The ASCO 2021 annual meeting conference will be held from June 4 to June 8, 2021, and while many abstracts have been released on the website, some are still under wraps