
ASCO 2021 Preview - Showstoppers in Gastric Cancers
- Home
- asco conference 2021
- showstoppers in gastric cancers
Showstoppers in Gastric Cancers - ASCO 2021
While ASCO 2021 annual meeting is around the corner, many big pharma giants are eagle eyeing this opportunity to present their data readouts from different oncology indications that could impact the treatment patterns in the near future. Of such indications, GI cancers have been hugely acknowledged and gained much importance over the past few years among such key companies. The top players presenting in the upcoming gastric ASCO 2021 session are Bristol Myers Squibb (BMS), Merck, BeiGene/Novartis, Daichi Sankyo, Astellas and AstraZeneca.
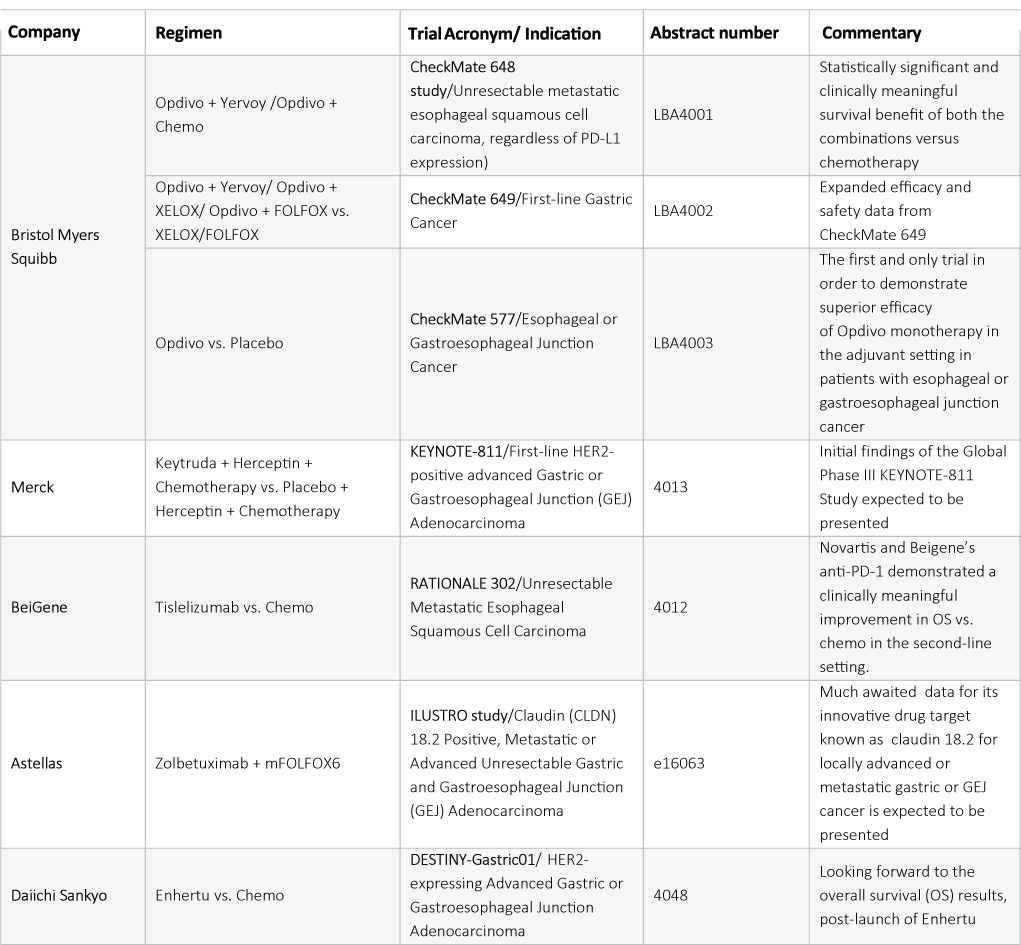
The first quarter of 2021 has been fortunate enough for Bristol Myers Squibb (BMS) and Merck as both are presenting their clinical data based on which their respective drugs got approved. In April 2021, the US FDA approved BMS” Opdivo (nivolumab) combined with chemotherapy for advanced or metastatic gastric cancer, gastroesophageal junction cancer (GEJ), and esophageal adenocarcinoma based on Phase III CheckMate-649 trial. This marked the first FDA-approved immunotherapy for the first-line treatment of gastric cancer. Following Nivolumab”s approval, Merck”s Keytruda (pembrolizumab) also hit the mark in May 2021, receiving accelerated approval in combination with Roche”s Herceptin (trastuzumab) and chemotherapy for the first-line treatment of patients with locally advanced unresectable or metastatic HER2 positive gastric or GEJ adenocarcinoma based on the prespecified interim analysis from the ongoing KEYNOTE-811 (NCT03615326) trial. Despite the prior Keytruda-chemo combo flop in newly diagnosed patients, Merck managed to sweep through this time.
Following the success of the Opdivo-Chemo combo, BMS has planned to disclose data from its Phase III CheckMate 648 study evaluating Opdivo plus Yervoy (ipilimumab) or Opdivo combined with chemotherapy versus chemotherapy in subjects with unresectable advanced, recurrent, or metastatic previously untreated esophageal squamous cell carcinoma (SCC). In April 2021, BMS announced the positive topline results from the pivotal study and, excitingly, now BMS is all geared up to present survival benefits of both the combinations in the upcoming ASCO 2021.
As BMS wants to leave no stones unturned to showcase its leading candidate in GI cancers, it has added one more Opdivo presentation in its bucket for the upcoming conference – data from its Phase III CheckMate 577 study evaluating adjuvant nivolumab or placebo in subjects with resected esophageal, or GEJ cancer. Earlier this year, Nivolumab”s BLA has already been accepted by the FDA along with a Priority Review and an assigned Prescription Drug User Fee Act (PDUFA) goal date of May 20, 2021. As this marks the first and only trial in order to demonstrate superior efficacy of Opdivo monotherapy in the adjuvant setting in patients with esophageal or gastroesophageal junction cancer, we expect this to create immense acclaim among oncologists, who can redefine the treatment choices in this setting.
BeiGene is leading the game in the second-line setting and is ready to showcase initial data from its Phase III RATIONALE 302 trial evaluating tislelizumab (BGB-A317), anti-PD-1 antibody, versus chemotherapy as second-line treatment in patients with advanced unresectable/metastatic esophageal SCC. This anti-PD-1 is already approved in China for certain patients with classical Hodgkin”s lymphoma and metastatic urothelial carcinoma, therefore having an edge on the safety and efficacy parameters known. Earlier this year, tislelizumab demonstrated a clinically meaningful improvement in overall survival (OS) in the intention-to-treat (ITT) population when compared to chemotherapy in unresectable/metastatic esophageal SCC. What is more exciting is that following the remarkable results in this target pool, Novartis immediately in-licensed the drug in North America, Europe, and Japan to further tap the potential of this uniquely designed anti-PD-1.
In the maintenance setting, AstraZeneca will be showcasing primary analysis from the PLATFORM trial evaluating maintenance durvalumab after first-line platinum-based chemotherapy in advanced oesophago-gastric (OG) adenocarcinoma.
Apart from much awaited and exciting results from the above mentioned anti-PD-1/PD-L1 immunotherapies, we cannot overlook the established efficacy of antibody-drug conjugates (ADC) in GI cancers. The beginning of this year marked the approval of Daichi Sankyo”s Enhertu for adult patients with locally advanced or metastatic HER2-positive gastric or GEJ adenocarcinoma who have received a prior trastuzumab-based regimen, and now the company plans to unveil the study”s final OS results. Following Daichi, Astellas has also planned to reveal data from its Phase II ILUSTRO study cohort II evaluating zolbetuximab plus mFOLFOX6 in claudin 18.2-positive locally advanced or metastatic gastric or GEJ cancer. The company is also evaluating the asset for pancreatic adenocarcinoma and is under clinical development phase.
Indeed, 2021 is replete with drugs covering various GI cancer indications, which might pertain to new approvals following ASCO 2021. The information for the same is summarized in the table below:
Executive Summary
The top players presenting in the upcoming gastric ASCO 2021 session are Bristol Myers Squibb (BMS), Merck, BeiGene/Novartis, Daichi Sankyo, Astellas and AstraZeneca.