All Posts
Pompe Disease: Causes, Symptoms, Diagnosis, Pathology, Current Marketed and Emerging Drugs in the Pipeline
Pompe disease is an inherited disorder caused by the buildup of a complex sugar called glycogen in the body's cells. The accumulation of glycogen in certain organs and tissues, especially muscles, impairs their ability to function normally. There are three types of Pompe disease, which differ in severity and the ag...
Find More
Sky Medical’s Geko™ device; Fist Assist Devices obtains Breakthrough Device designation; Edwards obtains FDA approval; BD launched instrument for testing; GE acquires BK Medical; Zynex’ CM-1500; Foldax’s TRIA biopolymer heart valve
Sky receives FDA clearance to market the geko™ device for venous insufficiency and ischemia On December 16, 2021, Sky Medical Technology Ltd, a medical device company, got approval from the US Food and Drug Administration (FDA) 510(k) to market the geko™ device for growing microcirculatory bl...
Find More
Major Highlights of Diffuse Large B-cell Lymphoma/ Large B-cell lymphoma (LBCL) in ASH 2021
ASH 2021,which ended on December 14, 2021, was a premier event in malignant and non-malignant hematology. Like every year, key pharma players took center stage and shared new data regarding the development of their primary candidates in cancer and blood disorders, showcasing the impact of ongoing research. DelveIns...
Find More
Major Highlights of Follicular Lymphoma (FL) in ASH 2021
ASH 2021,which ended on December 14, 2021, was a premier event in malignant and non-malignant hematology. Like every year, key pharma players took center stage and shared new data regarding the development of their primary candidates in cancer and blood disorders, showcasing the impact of ongoing research. DelveIns...
Find More
Analyzing the Key Trends Driving the Biosimilar Market Growth
The Biosimilars market is growing at a breakneck pace. The biosimilar market provides a compelling economic value proposition. This has been a considerable element in expanding the biosimilar market, and the future of this sector appears promising. Many pharmaceutical companies from across the globe are proactively...
Find More
Edwards’ Sapien 3 with Alterra Prestent; Koios Medical’s breast, thyroid cancer-spotting AI; Lineage Collaborates with Genentech; Novartis, BeiGene ink deal
Edwards secures FDA approval for Sapien 3 with Alterra Prestent for Transcatheter Pulmonic Valve Replacement Edwards Lifesciences declared to receive approval from the U.S. Food and Drug Administration (FDA) to use the Edwards SAPIEN 3 transcatheter valve with the Alterra adaptive prestent (SAPIEN 3 with Alterra...
Find More
Pertussis Vaccines: Whole-cell & Acellular Vaccines, Different Types & Manufacturers along with the Regimen in Various Age Groups
Pertussis, also commonly known as whooping cough, is a very highly contagious respiratory disease. It is caused by the bacterium Bordetella Pertussis. Pertussis can be a serious disease for people of all ages but especially for babies. Pertussis vaccines offer the best protection against this very contagious diseas...
Find More
What is Driving the Pertussis Treatment Market Forward?
Pertussis, which was first discovered in the Paris epidemic of 1578, is distinguished by a 2-week paroxysmal cough, an inspiratory whoop, posttussive emesis, and posttussive syncope. Pertussis is caused by Bordetella pertussis. In 1906, Bordetella pertussis was discovered, and in the 1940s, a vaccine was created. P...
Find More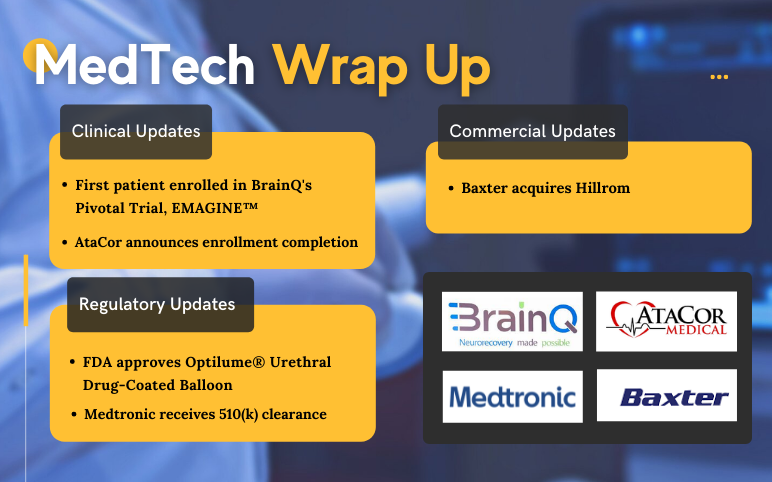
BrainQ’s EMAGINE Trial; AtaCor’s EV-ICD study; Urotronic’s Optilume Urethral Drug-Coated Balloon approval; Medtronic receives 510(k) clearance; Baxter acquires Hillrom
First patient enrolled in BrainQ's Pivotal Trial, EMAGINE™, for frequency-tuned electromagnetic field therapy to reduce disability following Ischemic Stroke On December 09, 2021, BrainQ announced the enrollment of the first patient at MedStar National Rehabilitation Hospital in its pivotal, randomized, ...
Find More
Insights into some of the Biosimilar Drugs that are approved and launched in 2021
Since the first biosimilar drug was approved in 2006, the EU has led the way in biosimilar regulation. Over the last decade, the EU has authorized the most biosimilars globally, gathering significant expertise with their usage and safety. Over ten years of clinical experience has shown that biosimilars licensed by ...
Find MoreEditor's Pick
Newsletter/Whitepaper
ASCO Conference 2023

The American Society of Clinical Oncology (ASCO) is one of the largest and most respected conferences in the field of oncology. Held annually, this conference brings together researchers, physicians, and other healthcare professionals from around the world to discuss the latest advances in cancer research, diagnosis, and treatment.






-Agonist.png)

