Search by Categories
- Insights
- Rare Disease
- Oncology
- Cell and Gene Therapy
- Medical Devices
- Consulting
- News & Analysis
-
Therapeutic Areas

Our expertise in the industry
Mindray Launched Groundbreaking 2-in-1 Handheld Ultrasound Device with Multi-device Connectivity On September 7, 2023, Mindray, a global leader and developer of healthcare solutions and techn...

Bristol Myers Squibb’s Investigational LPA1 Antagonist Reduces Rate of Lung Function Decline in Progressive Pulmonary Fibrosis Cohort of Phase II Study BMS-986278, a potential first-in-class oral lysophosphatidic acid receptor 1 (LPA1) antagonist, was studied in patients with progressive pulmonary fibrosis (PPF)...
Find More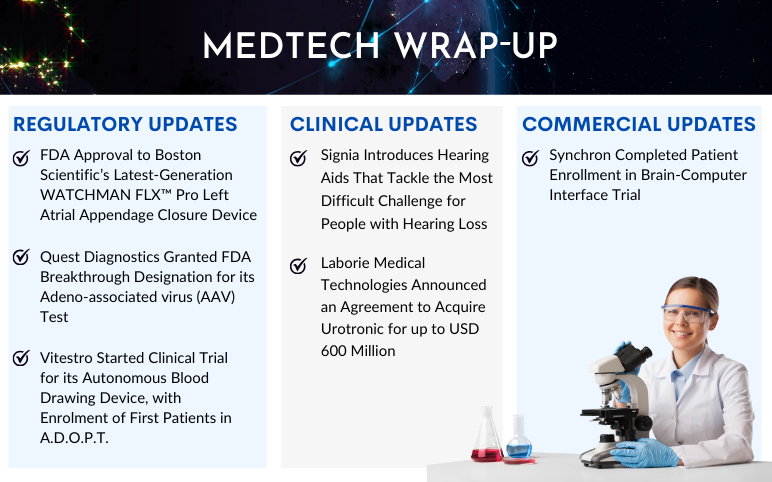
Quest Diagnostics Granted FDA Breakthrough Designation for its Adeno-associated virus (AAV) Test On August 30, 2023, Quest Diagnostics announced that its AAVrh74 ELISA assay (CDx) has been granted Breakthrough Device Designation from the US Food and Drug Administration (FDA). The enzyme-linked immunosor...
Find More
FDA Grants Breakthrough Therapy Designations to Trastuzumab Deruxtecan for HER2+ Solid Tumors, Including mCRC ENHERTU® (fam-trastuzumab deruxtecan-nxki) has been granted two additional Breakthrough Therapy Designations (BTDs) in the United States for the treatment of adult patients with unresectable or metastati...
Find More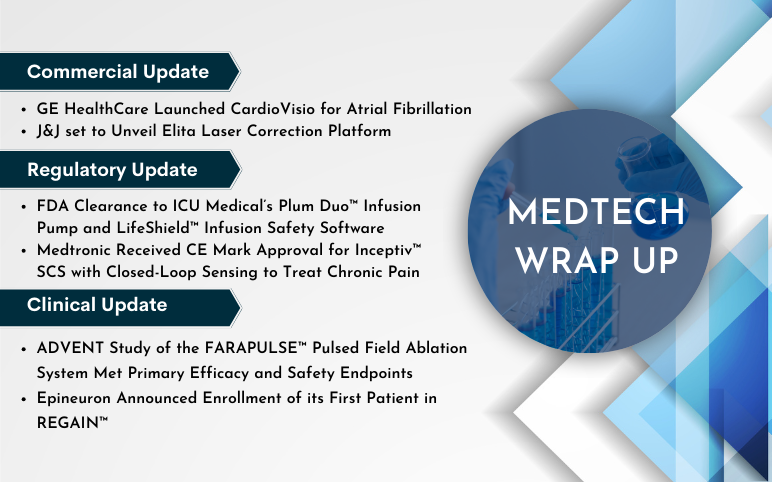
GE HealthCare Launched CardioVisio for Atrial Fibrillation, a Digital, Patient-Centric Clinical Decision Support Tool to Enable Precision Care On August 24, 2023, GE HealthCare launched CardioVisio for Atrial Fibrillation (AFib), a digital tool designed to assist clinicians in visualizing longitudinal data relev...
Find More
FDA Approves Bristol Myers Squibb’s Reblozyl as First-Line Treatment of Anemia in Adults with Lower-Risk MDS Who May Require Transfusions Bristol Myers Squibb announced that the Food and Drug Administration (FDA) has approved Reblozyl® (luspatercept-aamt) for the treatment of anemia in adult patients with very l...
Find More
Smith+Nephew Launched Hip Arthroplasty System in India On August 17, 2023, The London-based orthopedic device maker Smith+Nephew announced the launch of its OR3O dual mobility system for use in primary and revision hip arthroplasty in India. The dual mobility implants have a smaller-diameter femoral hea...
Find More
Eylea HD Injection 8 Mg Approved By FDA for Treatment of Wet AMD, DME, and Diabetic Retinopathy The FDA has approved Regeneron Pharmaceuticals’ EYLEA HD (aflibercept) Injection of 8 mg for the treatment of patients with wet age-related macular degeneration (wAMD), diabetic macular edema (DME), and diabetic retin...
Find More
4WEB Medical Launched its Cervical Spine Plating Solution On August 15, 2023, 4WEB Medical, an orthopedic implant company focused on developing innovative implants that utilize its proprietary Truss Implant Technology™, launched the newest addition to the company's implant portfolio, the Cervical Spine Pla...
Find More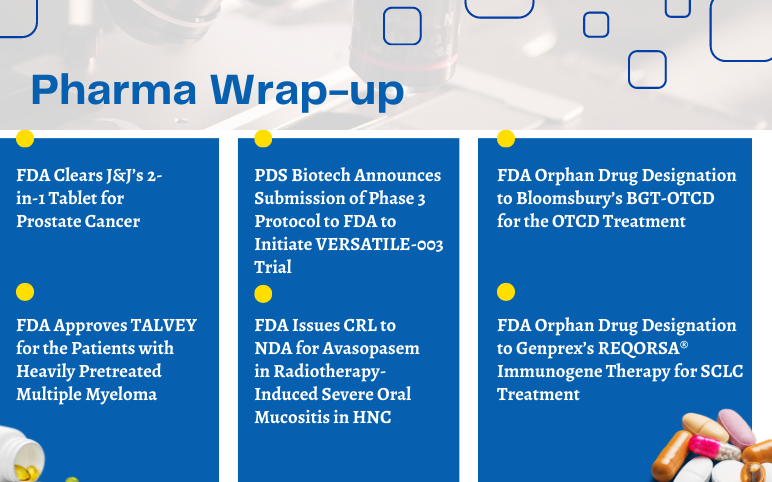
FDA Clears J&J’s 2-in-1 Tablet for Prostate Cancer Johnson & Johnson's Janssen Pharmaceutical Companies stated that the US Food and Drug Administration (FDA) had approved AKEEGA (niraparib and abiraterone acetate), the first-and-only dual-action tablet combining a PARP inhibitor including abiraterone ace...
Find More
Hemophilia, a genetic bleeding disorder characterized by a clotting factor protein deficiency. Intak.....
Find More
Major Depressive Disorder (MDD), also known as clinical depression, is a severe, debilitating and fr.....
Find More
Schizophrenia is a severe mental illness that interferes with a person's ability to think clearly, m.....
Find More
Scleroderma is a chronic disease that affects the skin, the connective tissue that supports and hold.....
Find More
Developmental and Epileptic Encephalopathy (DEE) refers to a group of severe epilepsies that are cha.....
Find More
Peanut Allergy accounts for the majority of severe food-associated allergic reactions. It is strictl.....
Find More
The American Society of Clinical Oncology (ASCO) is one of the largest and most respected conferences in the field of oncology. Held annually, this conference brings together researchers, physicians, and other healthcare professionals from around the world to discuss the latest advances in cancer research, diagnosis, and treatment.