Immune checkpoint inhibitors are drugs that inhibit immune checkpoints. These drugs allow immune cells to respond more strongly to cancer by blocking them. This blocks the “off” signal, allowing T-cells to kill cancer cells.
Checkpoint inhibitors can be used to treat a variety of cancers, including lung cancer (NSCLC and SCLC), melanoma, breast cancer (triple-negative, metastatic breast cancer), Hodgkin lymphoma, Urothelial carcinoma, Renal cell cancer (RCC), and others. Immune checkpoint inhibitors outperform other commonly used treatment therapies, such as chemotherapy, in terms of survival time and potency against cancer cells.
Nonetheless, a few side effects are associated with the use of immune checkpoint inhibitors that affect people in various ways. Furthermore, despite being a highly effective therapy option, there is a risk of developing resistance to checkpoint inhibitors because some patients do not respond at all (primary resistance; refractory) or stop responding over time (acquired resistance; relapsed).
There are few FDA-approved checkpoint inhibitors for lung cancer treatment, such as Non-Small Cell Lung Cancer (NSCLC). It is estimated that in 2021, approximately 235K new lung cancer cases were diagnosed in the United States, with 131K people dying from the disease.
According to the estimates based on DelveInsight analysis, the total 7MM incident cases of checkpoint-inhibitor-treated patients in 2021 were around 158K, out of which the highest cases were seen in the United States. The EU5 countries accounted for approximately 64K cases in 2021, and Japan had about 29K cases in 2021. The total incident cases of checkpoint-inhibitor-treated patients in 7MM are calculated by adding the cases in individual countries.
Moreover, in 2021, NSCLC had the highest incidence of IO-treated patients in the United States, with approximately 49K cases projected to increase by 2032.
Immunotherapy, as opposed to traditional cancer therapies such as chemotherapy, radiation therapy, or targeted therapies, is the most recent treatment option for many cancer types.
FDA-approved Checkpoint Inhibitor Drugs
Unlike chemotherapy, which targets cancerous tumors directly, immunotherapy, such as checkpoint inhibitors, treats patients by affecting their immune systems. The PD-1/PD-L1 and CTLA-4 pathways are two common checkpoints that these inhibitors affect. There are some FDA-approved checkpoint inhibitor drugs for specific cancers available in the checkpoint inhibitors market. Some checkpoint inhibitors are also used to treat tumors anywhere in the body by targeting specific genetic changes. These are referred to as “tumor-agnostic treatments.”
The FDA approved the first checkpoint immunotherapy treatment for any type of cancer in 2011 for metastatic melanoma. Yervoy (ipilimumab) was the first checkpoint inhibitor to be approved for melanoma treatment. Yervoy works by targeting the CTLA-4 pathway; it is approved for subsets of patients with melanoma, mesothelioma, liver cancer, and lung cancer.
The US Food and Drug Administration approved Opdivo (nivolumab) and Keytruda (pembrolizumab) to treat melanoma in 2014. Opdivo and Keytruda were later approved for use in various cancer types, including bladder cancer, colorectal cancer, head and neck cancer, kidney cancer, liver cancer, lung cancer, lymphoma, melanoma, and mesothelioma.
Atezolizumab (Tecentriq), a checkpoint inhibitor that targets the PD-1/PD-L1 pathway, was approved in 2016 for subsets of patients with bladder cancer, breast cancer, liver cancer, lung cancer, and melanoma. Cemiplimab (Libtayo) is another checkpoint inhibitor that targets the PD-1/PD-L1 pathway. It was approved in 2018 and is now used to treat subsets of patients with cutaneous squamous cell carcinoma and basal cell carcinoma.
Durvalumab (Imfinzi), another checkpoint inhibitor targeting the PD-1/PD-L1 pathway, is approved for certain subsets of patients with bladder cancer and lung cancer. Forbye, Erdafitinib (Balversa) was approved by the FDA in April 2019 as the first therapy targeting a genetic alteration to treat patients with metastatic urothelial carcinoma, the most common type of bladder cancer.
Furthermore, a plethora of companies, including Bristol-Myers Squibb, AstraZeneca, Merck, Genentech, Hoffmann-La Roche, Regeneron Pharmaceuticals, Janssen Research and Development, LLC, 4D pharma plc., 4SC AG, OncoSec Medical, Mirati Therapeutics, Ascentage Pharma Group, ENB Therapeutics, Inc., Exicure, Inc., Evelo Biosciences, Inc., Eisai, Kartos Therapeutics, Exelixis, ImmunityBio, Checkmate Pharmaceuticals, BerGenBio, CatalYm, and others are conducting various immune checkpoint inhibitors clinical trials to improve the refractory cancer treatment landscape.
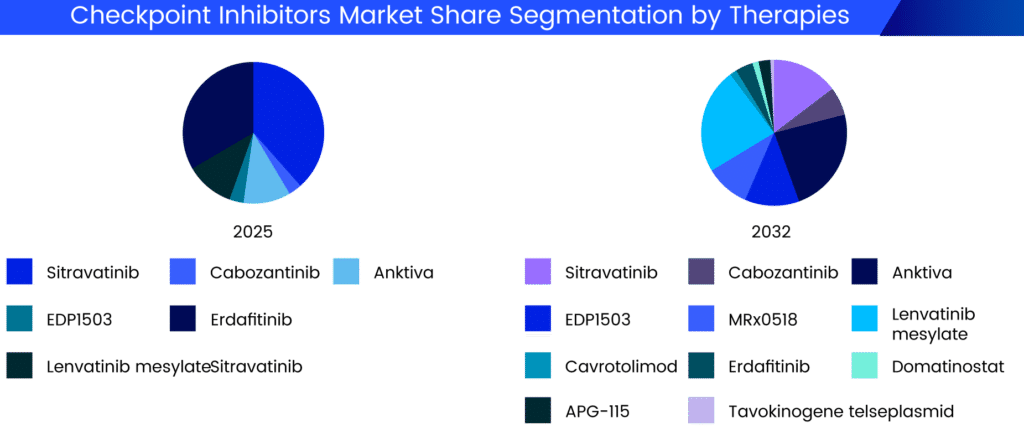
As per DelveInsight analysis, these checkpoint inhibitor drugs are expected to grab a significant chunk of the checkpoint inhibitors market. As a result, the checkpoint inhibitors market size will skyrocket in the coming years.
Promising Checkpoint Inhibitors in Development
The dynamics of the checkpoint inhibitor market are expected to change as companies around the world working tirelessly to develop new drug therapy options to treat a wide range of checkpoint inhibitor refractory cancers. Several checkpoint inhibitor drugs are in the pipeline for refractory cancer treatment. The promising checkpoint inhibitors in development include:
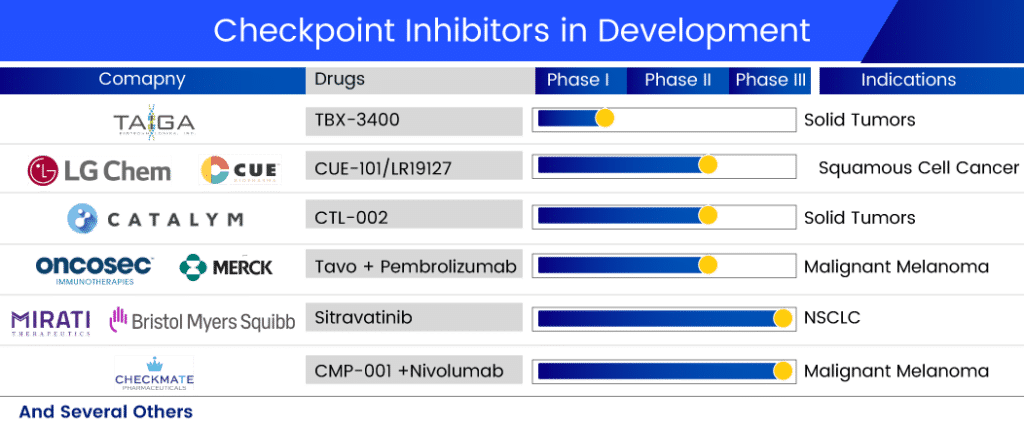
The lead product of Mirati, Sitravatinib, is a well-known multi-kinase inhibitor developed in collaboration with Bristol-Myers Squibb. This candidate is thought to inhibit the TAM family (TYRO3, AXL, and MER), the VEGF receptor type 2 (VEGFR2), and KIT. When Sitravatinib targets these receptor tyrosine kinases, it weakens cancer’s defenses in the tumor microenvironment, increasing the likelihood that cancer will respond to checkpoint inhibitor therapy. Currently, it is in Phase III trials for NSCLC treatment. A randomized phase III study of Sitravatinib in combination with Nivolumab vs. Docetaxel in patients with advanced non-squamous NSCLC who have progressed on or after platinum-based chemotherapy and checkpoint inhibitor therapy (2nd or 3rd line checkpoint refractory) is currently underway.
In collaboration with Merck Sharp & Dohme, OncoSec Medical is conducting a phase II trial for stage III/IV melanoma. TAVO is a DNA-based interleukin-12 (IL-12), a naturally occurring protein in the body that stimulates the immune system. It is delivered directly into the tumor via our patented electroporation (EP) gene delivery system, which uses a series of brief energy pulses. This drug has been designated as a fast-track and orphan drug for metastatic melanoma treatment.
Checkmate Pharmaceuticals and IQVIA Biotech are also working together on a project. The companies are running a Phase II trial with CMP-001 (Vidutolimod) and Nivolumab. The safety, efficacy, and tolerability of CMP-001 administered by intratumoral (IT) injection in combination with nivolumab in subjects with refractory unresectable or metastatic melanoma will be assessed in this trial. The growing body of promising clinical data for CMP-001 shows that it has the potential to address an unmet need for patients whose cancers are resistant to immunotherapeutic agents. This multicenter, open-label study with 100 participants is expected to be finished in February 2023.
In 2021, the company announced the Phase Ib study of the Toll-like receptor 9 agonist vidutolimod pembrolizumab intratumoral injection in patients with PD-1 blockade-refractory melanoma. Trials for advanced melanoma (Phase II and Phase II/III) and HNSCC (Phase II) are currently in the pipeline.
CatalYm is developing CTL-002 in Phase II. The company announced in January 2021 that the first patient was enrolled and safely treated in the first clinical trial with CTL-002, abbreviated GDFATHER, in December 2020. GDFATHER is a multicenter, open-label clinical trial evaluating CTL-002 intravenous (IV) administration as monotherapy and in combination with an anti-PD-1 checkpoint inhibitor. Patients with advanced-stage solid tumors who have relapsed or are refractory to previous anti-PD-1/PD-L1 treatments are being sought for the trial. The company presented the results of its PhasePhase I trial plenary session at the AACR-NCI-EORTC conference in September 2021. The company announced the treatment of the first patients in a series of Phase IIa cohorts targeting solid tumors in March 2022, kicking off CTL-002’s Phase II development.
TBX-3400, a Taiga product, is currently being tested in human clinical trials as a treatment for various solid tumors due to its agnostic nature. A Phase I single-center dose-escalation study is currently underway to assess the safety, tolerability, and early efficacy of TBX-3400 in patients with Stage III and IV melanoma resistant to immune checkpoint inhibitors.
LGChem is also developing CUE-101/LR19127 in collaboration with Cue Biopharma for the treatment of HPV+ recurrent or metastatic head and neck cancer patients with confirmed progressive disease who are refractory or resistant to first-line platinum-based chemotherapy and CPIs. CUE-101 is a fusion protein that targets Human Papillomavirus 16 (HPV16)-driven cancers by activating and expanding tumor-specific T cells directly in the patient’s body. This drug is currently in Phase II development, and LGChem owns the Asia rights to this checkpoint inhibitor drug. Cue Biopharma announced monotherapy registration study potential based on mOS in July 2022, with a decision expected in Q4 2022 or Q1 2023. In addition, the company intends to conduct a combination therapy registration study in the second half of 2023.
What Lies Ahead for Checkpoint Inhibitors?
Recent FDA approvals of anti-PD-1 regimens in combination with anti-CTLA-4 or VEGF tyrosine kinase inhibitors are reshaping front-line therapy for metastatic kidney cancer. In the meantime, therapeutics targeting programmed death ligand 1 (PD-L1), one of the two major PD-1 ligands, are being researched. Surprisingly, not all PD-1 and PD-L1 agents produce comparable clinical outcomes, which could be attributed to biological differences in the cellular expression and regulation of these targets.
As a result, the question of the next best option remains if the patient (without driver mutation) progresses after I-O treatment or is found to be a non-responder. Currently, the answer in such cases is platinum-based chemotherapy with or without other therapies (in case of NSCLC). These therapies are primarily reserved for patients who have progressed after receiving PD-L1 inhibitors in the first-line setting and those who receive it in the second-line setting if they did not receive it in the first-line setting.
Extensive genomic studies have aided in identifying molecular biomarkers and directed the beginning of targeted therapies as an effective additional option, driving the evolution of cancer treatment. Checkpoint inhibitors have the potential to enable long-term immunity against cancer cells while also increasing survival expectations.
Despite being a highly effective therapy option, there is a risk of developing resistance to immunotherapies because some patients do not respond from the start or stop responding over time. For example, even though PD-1 or PD-L1 have been at the forefront of immunotherapy in several cancer types, nearly half of patients with PD-L1 positive tumors show resistance or relapse following PD-1/PD-L1 blockade. As a result of this significant unmet need in society, certain pharmaceutical companies are attempting to develop checkpoint inhibitors that will be useful in treating the refractory patient pool. Numerous targets have shown promise as resistance biomarkers and as combination therapy targets, and it has also been demonstrated that primary resistance can be overcome by targeting related pathways.

FAQS
Immune checkpoint inhibitors are drugs that inhibit immune checkpoints. These drugs allow immune cells to respond more strongly to cancer by blocking them. This blocks the “off” signal, allowing T-cells to kill cancer cells.
Checkpoint inhibitors can be used to treat a variety of cancers, including lung cancer (NSCLC and SCLC), melanoma, breast cancer (triple-negative, metastatic breast cancer), Hodgkin lymphoma, Urothelial carcinoma, Renal cell cancer (RCC), and others.
Yervoy (ipilimumab), Opdivo (nivolumab) and Keytruda (pembrolizumab), Atezolizumab (Tecentriq), Cemiplimab (Libtayo), Durvalumab (Imfinzi), Erdafitinib (Balversa), and others are some of the checkpoint inhibitor drugs approved by the FDA.
A few side effects are associated with the use of immune checkpoint inhibitors that affect people in various ways. Furthermore, despite being a highly effective therapy option, there is a risk of developing resistance to checkpoint inhibitors because some patients do not respond at all (primary resistance; refractory) or stop responding over time (acquired resistance; relapsed).
A plethora of companies, including Bristol-Myers Squibb, AstraZeneca, Merck, Genentech, Hoffmann-La Roche, Regeneron Pharmaceuticals, Janssen Research and Development, LLC, 4D pharma plc., 4SC AG, OncoSec Medical, Mirati Therapeutics, Ascentage Pharma Group, ENB Therapeutics, Inc., Exicure, Inc., Evelo Biosciences, Inc., Eisai, Kartos Therapeutics, Exelixis, ImmunityBio, Checkmate Pharmaceuticals, BerGenBio, CatalYm, and others are conducting various immune checkpoint inhibitors clinical trials to improve the refractory cancer treatment landscape.








-Agonist.png)


