Search by Categories
- Insights
- Rare Disease
- Oncology
- Cell and Gene Therapy
- Medical Devices
- Consulting
- News & Analysis
-
Therapeutic Areas

Our expertise in the industry
As we step into the crisp corridors of 2024, the healthcare landscape unfolds a compelling saga of mergers, strategic funding, and transformative acquisitions. In this month-by-month analysis, we d...

4DMT Presents Positive Interim Data from Randomized Phase II PRISM Clinical Trial of Intravitreal 4D-150 Demonstrating Favorable Tolerability & Clinical Activity in Wet AMD 4D Molecular Therapeutics, a prominent company in the field of genetic medicines with a focus on harnessing the full potential of geneti...
Find More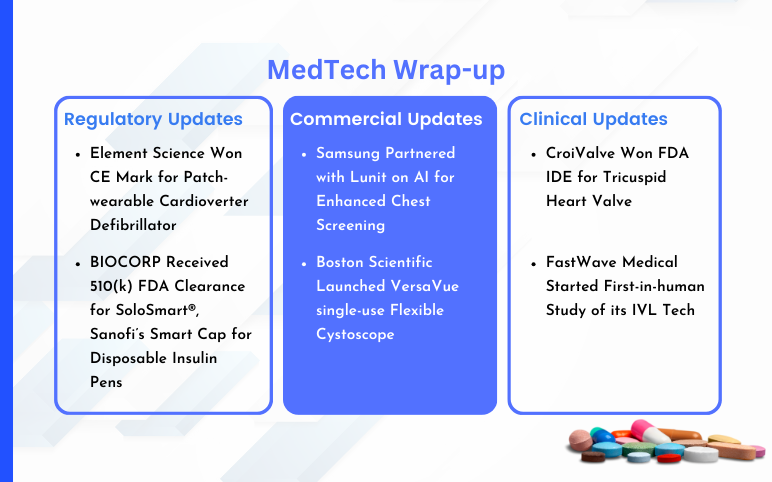
Samsung partnered with Lunit on AI for Enhanced Chest Screening On January 25, 2024, Samsung entered into a supply collaboration with Lunit, in order to employ its AI-powered technology for conducting chest screenings. The agreement was signed by Boston Imaging, which serves as the U.S. hub for Samsung's d...
Find More
Merck’s KEYTRUDA Reduced the Risk of Death by 38% Versus Placebo as Adjuvant Therapy for Patients With Renal Cell Cancer (RCC) at an Increased Risk of Recurrence Following Nephrectomy Merck, also known as MSD beyond the United States and Canada, has revealed findings from the Phase III KEYNOTE-564 trial, which a...
Find More
Pi-Cardia Receives FDA Breakthrough Device Designation for ShortCut™ Pi-Cardia Ltd., a prominent player in advancing catheter-based leaflet modification solutions for heart valve treatment, revealed that its ShortCut™ device has attained Breakthrough Device Designation from the US Food and Drug Administration. S...
Find More
Opdivo in Combination with CABOMETYX Demonstrates Long-Term Survival Benefits After Four Years of Follow-Up in the CheckMate -9ER Trial in First-Line Advanced Renal Cell Carcinoma Bristol Myers Squibb and Exelixis, Inc. have released the four-year follow-up findings from the CheckMate -9ER trial, which investiga...
Find More
Bayer’s AskBio initiates Phase II GenePHIT trial in Congestive Heart Failure Merck, also recognized as MSD in regions beyond the United States and Canada, has officially announced that the FDA has approved for the use of KEYTRUDA, Merck's anti-PD-1 therapy, in conjunction with chemoradiotherapy (CRT) for treatin...
Find More
Medtronic Received CE Mark for its Next Generation Micra Leadless Pacing Systems On January 5, 2024, Medtronic announced that the Micra AV2 and Micra VR2, the next generation of its industry-leading tiny, leadless pacemakers, earned the CE (Conformité Européenne) Mark. The world's tiniest pacemakers, the rMic...
Find More
Lutikizumab Showed Positive Results in a Phase II Trial of Adults with Moderate to Severe Hidradenitis Suppurativa as Program Advances to Phase III AbbVie has reported the results of Phase II trials indicating that adults experiencing moderate to severe hidradenitis suppurativa, and who had previously not respon...
Find More
HR Pharmaceuticals, Inc. Received Exclusive Commercialization Rights to Poiesis Medical’s Dual Balloon Catheter Technology in North America On December 28, 2023, HR Pharmaceuticals entered into a collaboration with Poiesis Medical LLC, to license Poiesis's Dual Balloon Catheter (Duette™). According to the terms ...
Find More
Tinnitus is a condition where a person experiences different kinds of noises in one or both ears. It.....
Find More
Rheumatoid Arthritis (RA) is an inflammatory disorder that affects an individual's single or multipl.....
Find More
Uremic Pruritusis also known as chronic kidney disease-associated pruritus (CKD-aP), is a chronic it.....
Find More
Atopic Dermatitis is a chronic inflammatory disease of the skin, which causes the skin to turn red a.....
Find More.png)
Virtual clinical trials (VCTs) are site-less clinical trials, which utilizes technology along with t.....
Find More
Celiac disease is an autoimmune disorder against the gluten consumed that creates toxins, thereby, d.....
Find More
The American Society of Clinical Oncology (ASCO) is one of the largest and most respected conferences in the field of oncology. Held annually, this conference brings together researchers, physicians, and other healthcare professionals from around the world to discuss the latest advances in cancer research, diagnosis, and treatment.