Table of Contents
Proprotein convertase subtilisin/Kexin type 9 (PCSK9) affects LDL receptor degradation and decreases the clearance of circulating LDL particles, which is crucial in cholesterol metabolism. PCSK9 is predominantly generated by hepatocytes, with additional sources including the intestines and kidneys. PCSK9 reduces LDLR in hepatocytes by increasing their metabolism and eventual death, preventing the receptors from being broken down and allowing them to continue to lower blood cholesterol.
PCSK9 inhibitors indications include patients with familial hypercholesterolemia who are resistant to statins or have increased LDL-C levels while being on maximally tolerated statin therapy. The PCSK9 inhibitors are also approved for the treatment of existing cardiovascular disease to minimize the risk of myocardial infarction, stroke, and coronary revascularization.
Familial hypercholesterolemia is one of the most prevalent hereditary illnesses, affecting approximately 1 in every 200 persons. Familial hypercholesterolemia is a global concern since few individuals are discovered globally, and most are found too late, generally when they are in their 40s and already have heart disease. Mutations in three genes cause familial hypercholesterolemia: the LDL-receptor gene (LDLR), the apoB gene, and the proprotein convertase subtilisin/Kexin 9 gene. In patients with no causal mutation, a polygenic etiology for their elevated LDL-C level is most likely. HoFH has a few phenotypic clinical symptoms, such as large xanthomas, early progressive atherosclerotic CVD, and other life-threatening diseases.
According to the estimates based on DelveInsight analysis, the total prevalent cases of familial hypercholesterolemia were 3.1 million cases in the 7MM in 2021, with the US accounting for the highest prevalent cases. Moreover, as per the estimates, the total age group-specific diagnosed prevalent cases of familial hypercholesterolemia were highest for the age group 50-59 years, followed by 60-69 years and 40-49 years, respectively, in 2021 in the 7MM.
Pharmacologically, PCSK9 can be suppressed by monoclonal antibodies that bind and neutralize PCSK9 or by RNA-targeting medicines that include an RNA strand complementary to PCSK9 mRNA. It causes the formation of an RNA-induced silencing complex (RISC), degrading PCSK9 mRNA over an extended time and suppressing PCSK9 synthesis.
The most prevalent PCSK9 inhibitors side effects are minimal. As a PCSK9 inhibitor injection, it causes back discomfort and cold or flu symptoms. These PCSK9 inhibitors medications may potentially cause allergic responses in certain people.
PCSK9 Inhibitors Market Insights
Among the PCSK9 inhibitors list, there are currently two FDA-approved PCSK9 inhibitors, namely, evolocumab and alirocumab, available in the United States for adult patients to reduce the risk of myocardial infarction, stroke, and unstable angina requiring hospitalization in adults with established cardiovascular disease as an adjunct to diet, alone or in combination with other lipid-lowering therapies, for the treatment of adults with primary hyperlipidemia (including HeFH).
Evolocumab is also licensed as an adjuvant to diet and other LDL-lowering therapies in HoFH patients who require further LDL-C reduction. When coupled with statin therapy, the mAb PCSK9 inhibitors evolocumab and alirocumab lower LDL-C levels by around 60% and reduce the risk of severe vascular events. Both of these PCSK9 inhibitors drugs have the same PCSK9 inhibitors mechanism of action. Additionally, Alirocumab has been approved in more than 60 countries and launched in more than 30 countries, including the US, Japan, Germany, the UK, Spain, and others.

Moreover, major PCSK9 inhibitors trials have shown that PCSK9 inhibitors, when combined with statin-based therapy, reduce ASCVD risk even further, and their use may need to be confined to patients at the highest risk for ASCVD. Moreover, in 2015, the European Commission approved the commercialization of alirocumab and evolocumab.
Furthermore, Novartis’ Leqvio (inclisiran, KJX839) is the first and only small interfering RNA (siRNA) medication to lower LDL-C levels using an RNA interference (RNAi) mode of action. It has the potential to enhance outcomes for people with ASCVD. Inclisiran is a long-acting synthetic siRNA directed against PCSK9 that has been shown to reduce hepatic PCSK9 synthesis and LDL-C levels drastically. Despite being accepted in Europe, Leqvio has yet to be approved in the United States. Due to unresolved facility inspection-related problems, the FDA announced that the agency could not approve the NDA by the Prescription Drug User Fee Act (PDUFA) action date of December 23, 2020. However, the European Commission authorized Leqvio (inclisiran) for the treatment of individuals with hypercholesterolemia or mixed dyslipidemia in December 2020.
Forbye, the dynamics of the PCSK9 inhibitors market are anticipated to change in the coming years due to several factors such as the improvement in the rise in the number of healthcare spending globally and rising cases of statin resistance in patients with familial hypercholesterolemia as well increasing risk of cardiovascular conditions such as myocardial infarction, coronary revascularization, etc.
The Future PCSK9 Inhibitors Market Looks Promising
Several leading pharmaceutical big names such as LIB Therapeutics, Ionis Pharmaceuticals, AstraZeneca, CiVi Biopharma, Merck & Co., Verve Therapeutics, and others are currently involved in developing PCSK9 inhibitors drugs. The emerging therapies for PCSK9 inhibitors in development will provide efficient therapeutic approaches. These emerging therapies include:
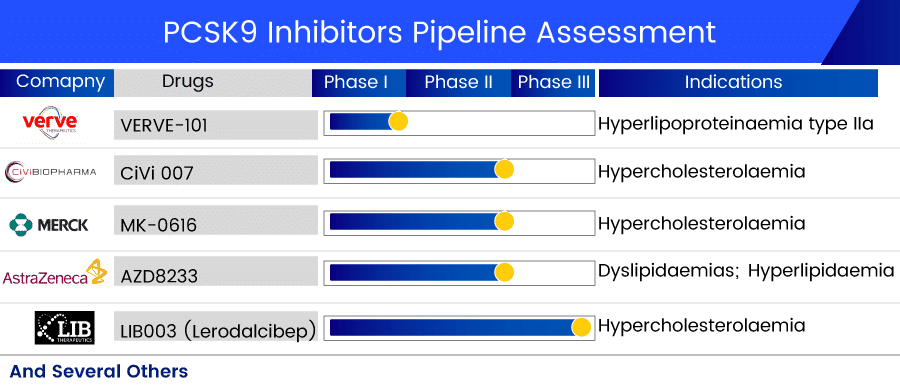
LIB003 (Lerodalcibep) by LIB Therapeutics is a subcutaneously given PCSK9 Inhibitor. The PCSK9 protein is an essential regulator of circulating LDLC levels (LDLR) through its inhibitory impact on LDL receptor recycling. The LDLR on the cell surface of the liver binds to LDL, and the LDLR-LDL complex is subsequently internalized. The LDLR is then recycled back to the cell surface up to 150 times. LIB003 is currently in Phase III PCSK9 inhibitors clinical trials. LIB003 has previously completed one Phase I, two Phase II, and six Phase III clinical investigations for cardiovascular diseases, risks, and cholesterolemia.
AZD8233 (ION449), originally IONIS-AZ4-2.5-LRx, is an experimental ligand-conjugated antisense medication. AstraZeneca is developing ION449 as part of a cooperation with Ionis Pharmaceuticals. It is a novel, investigational ASO medication that is administered subcutaneously once a month to lower blood cholesterol levels in hypercholesterolemic individuals by suppressing PCSK9 expression in the nucleus. AZD8233, through decreasing PCSK9 levels, is intended to increase the number of LDL receptors accessible to remove LDL from the blood, lowering LDL-C levels. AZD8233 is currently in Phase II PCSK9 inhibitors clinical trials for dyslipidemia treatment, including hyperlipidemia.
Merck’s MK-0616 is an orally bioavailable PCSK9 inhibitor with nonclinical pharmacokinetic and safety features that warrant a clinical study. Merck has completed two Phase I trials on the safety and effectiveness of this candidate for cholesterol-lowering and evaluated the decrease of high LDL cholesterol levels. This PCSK9 inhibitors medication is now being studied in the Phase II trial for familial hypercholesterolemia treatment.
Verve Therapeutics’ lead asset, VERVE-101, is designed to switch off the PCSK9 gene in the liver, disrupting blood PCSK9 protein synthesis and thereby lowering blood LDL-C levels, to reduce a patient’s risk of atherosclerotic cardiovascular disease. VERVE-101 is now being studied in Phase I for heterozygous familial hypercholesterolemia treatment, a potentially deadly hereditary heart condition.
CiVi 007 is a long-acting, third-generation PCSK9 inhibitor in development by CiVi Biopharma for hypercholesterolemia treatment and the prevention of atherosclerotic cardiovascular disease (ASCVD). CIVI 007 has the potential to combine mAb-like LDL effectiveness with better dosing ease and a cheap cost of goods, according to the company. CiVi 007 decreases LDL cholesterol by employing cutting-edge technology to inhibit the development of a critical enzyme, PCSK9, at the mRNA level. CiVi-007 just completed one Phase I and one Phase II clinical study in 2020.
CiVi-008 is currently being developed as an oral therapy for hypercholesterolemia and the prevention of atherosclerotic cardiovascular disease. It is now in the preclinical development stage, and the company intends to begin clinical trials for this PCSK9 inhibitors medication soon.
PCSK9 Inhibitors Market: Way Ahead
PCSK9 inhibition in a preventive scenario can alleviate the CVD burden and will favor the PCSK9 inhibitors market. Furthermore, the implications of PCSK9 inhibitors in larger therapeutic areas will help in the expansion of the PCSK9 Inhibitors market. Though monoclonal antibodies have established themselves as the most therapeutically beneficial PCSK9 inhibitors, for the time being, additional novel therapies are currently in various stages of research and will be accessible in the PCSK9 inhibitors market shortly. The anticipated launch of these therapies will boost the PCSK9 inhibitors market in the coming years.
According to DelveInsight analysis, the total PCSK9 inhibitors market size was approximately USD 1,309 million in the 7MM in 2021, which is expected to rise during the forecast period (2022–2032).
However, despite manufacturers laboring for years to gather proof, reimbursement limits for the two PCSK9 inhibitors medications remain in large European PSCK9 inhibitors markets such as France, the United Kingdom, Italy, Germany, and Spain. Furthermore, commercial payer activities impact PCSK9 inhibitor market access and Repatha sales. Additionally, the usage of off-label therapies and generics will give a threat to the growth of the PCSK9 inhibitors market in the future.
FAQs
PCSK9 controls the degradation of the LDL receptor in response to cholesterol concentrations within the cell. PCSK9 binds to an extracellular portion of the LDL receptor. Apolipoprotein-B100, the structural protein of LDL and a ligand for the LDL receptor, interacts with a distinct location on the LDL receptor.
Inhibiting PCSK9 causes more LDL receptors to be recycled to the cell’s surface, which should boost the clearance of LDL cholesterol from circulation. Several techniques for pharmacological inhibition of PCSK9 have been studied.
PCSK9 inhibitors are currently being used to treat individuals with familial hypercholesterolemia who are statin-intolerant or have increased LDL-C levels while being on maximally tolerated statin therapy. The inhibitors are also licensed to minimize the risk of myocardial infarction, stroke, and coronary revascularization in patients with existing cardiovascular disease.
Several leading pharmaceutical big names such as LIB Therapeutics, Ionis Pharmaceuticals, AstraZeneca, CiVi Biopharma, Merck & Co., Verve Therapeutics, and others are currently involved in developing PCSK9 inhibitors drugs.
Among the PCSK9 inhibitors list, there are currently two FDA-approved PCSK9 inhibitors, evolocumab and alirocumab, available in the United States.








-Agonist.png)


