Search by Categories
- Insights
- Rare Disease
- Oncology
- Cell and Gene Therapy
- Medical Devices
- Consulting
- News & Analysis
-
Therapeutic Areas

Our expertise in the industry

Dermira pulling plug on acne drug after phase 3 failure Pharmaceutical company Dermira is pulling the plug on its acne drug (olumacostat glasaretil), after it failed to meet its primary endpoints in a pair of phase 3 trials. The drug was tested in patients aged nine and older with moderate to severe acne vulgaris. ...
Find More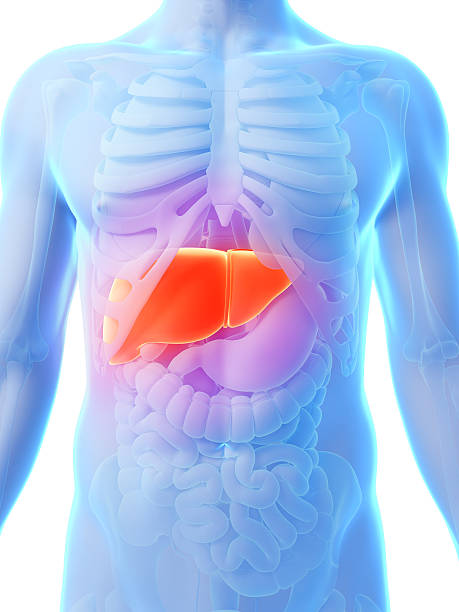
Obesity and diabetes are a global epidemic contributing to an increasing prevalence of related systemic disorders, including non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH). Nonalcoholic Steatohepatitis (NASH) is a subtype of NAFLD (Nonalcoholic Fatty Liver disease) that is charact...
Find More
NASH is defined as the most common form of Nonalcoholic fatty liver disease (NAFLD). The disease is characterized by a spectrum of symptoms including simple steatosis, increased levels of fibrosis, and cirrhosis of the liver. NASH can also result in advanced fibrosis, NASH cirrhosis, and NASH Decompensated Cirrhosi...
Find More
Gilead Sciences Inc. bought Kite Pharma (USD 11.9 Billion) while Celgene took over Juno Therapeutics (USD 9 Billion) to make the greatest speculation into this rising subject over the recent one year. While experts hope that each individual CAR-T therapies will yield sales of USD 1 billion, the investing companies...
Find More
Ionis pharmaceuticals recently licensed-out its antisense drug to AstraZeneca in deal total worth up to USD 330 million Ionis Pharmaceuticals has recently licensed-out an antisense drug to AstraZeneca, bagging a payment of USD 30 million as an upfront licensing fee. AstraZeneca will now be taking care of the develo...
Find More
Rice University researchers tested CRISPR to cure sickle cell, now preparing for upcoming challenges A team of Researchers led by Rice University has recently tested CRISPR to correct the sickle cell mutation in up to 40% of the total stem cell samples taken from affected patients. Although the results are quite pro...
Find More
Immune checkpoints are the regulators of immune activation. They play a key role in maintaining immune homeostasis and preventing autoimmunity. Immune checkpoint activators are small molecules responsible for maintenance, modulation, and regulation of immune responses. Its significance in immune-therapeutics is back...
Find More
Vertex pharmaceutical’s growth story in biotech with Symdeko’s approval FDA has recently approved Vertex Pharmaceutical’s latest combination treatment, Symdeko, to serve the cystic fibrosis patient population. Symdeko's annual list price has been set at USD 292,000. Symdeko has paired with a fresh corrector, tezacaf...
Find More
Gilead Sciences has recently launched a highly effective hepatitis C drug Sovaldi at a whopping price of USD 84,000 per treatment course at the same time when ‘How to price a cure’ discussion has entered the pharma industry. Gilead bought a launch-ready drug and launched it very quietly hoping that it's very cost-ef...
Find More
Roivant expanding its horizon into metabolic diseases segment with USD 650 million diabetes pact with Poxel Roivant Sciences has recently signed a USD 650 million deal to acquire near-global rights to Poxel pharma’s phase 3-ready Type 2 diabetes drug (imeglimin). It’s been a while since Poxel was waiting f...
Find More
The American Society of Clinical Oncology (ASCO) is one of the largest and most respected conferences in the field of oncology. Held annually, this conference brings together researchers, physicians, and other healthcare professionals from around the world to discuss the latest advances in cancer research, diagnosis, and treatment.