Search by Categories
- Insights
- Rare Disease
- Oncology
- Cell and Gene Therapy
- Medical Devices
- Consulting
- News & Analysis
-
Therapeutic Areas

Our expertise in the industry

Medtronic Launches World's First and Only Infusion Set for Insulin Pumps that Doubles Wear Time up to 7 days in the US On November 15, 2022, Medtronic plc, a global leader in healthcare technology, announced the US launch of the Medtronic Extended infusion set, the first and only infusion set labeled for up to ...
Find More
Diabetes is a chronic metabolic disorder that is influenced by a wide variety of factors and is characterized by high or low blood glucose (or blood sugar) levels in the body. Diabetes is a major risk factor for blindness, kidney failure, heart attacks, stroke, and lower limb amputation. Also, it can significantly ...
Find More
CereVasc Announces FDA Approval of Second IDE Study of the eShunt® System On August 09, 2022, CereVasc, Inc., a privately held, clinical-stage medical device company developing novel, minimally invasive treatments for neurological diseases, announced that the U.S. Food and Drug Administration (FDA) has approved ...
Find More
It is estimated that around five billion people are on the verge of losing access to essential healthcare services by 2030, for example, mandatory healthcare services, necessary medicine, and visiting healthcare providers. This issue can further become complex if there is an increase in the shortage of trained heal...
Find More
Byondis Files its HER2-Targeting Antibody-Drug Conjugate (ADC) Trastuzumab Duocarmazine in the US and Europe Byondis has filed for clearance of its HER2-targeting antibody-drug conjugate (ADC) trastuzumab duocarmazine in the United States and Europe, setting up a battle with heavyweight competitors Roche and Ast...
Find More
Conformal Medical Announces Launch of CONFORM Pivotal Trial On June 17, 2022, Conformal Medical Inc, is a medical device company manufacturing devices to avoid strokes in patients with non-valvular atrial fibrillation and developing next-generation LAAO technology. Its exclusive technology is intended to make le...
Find More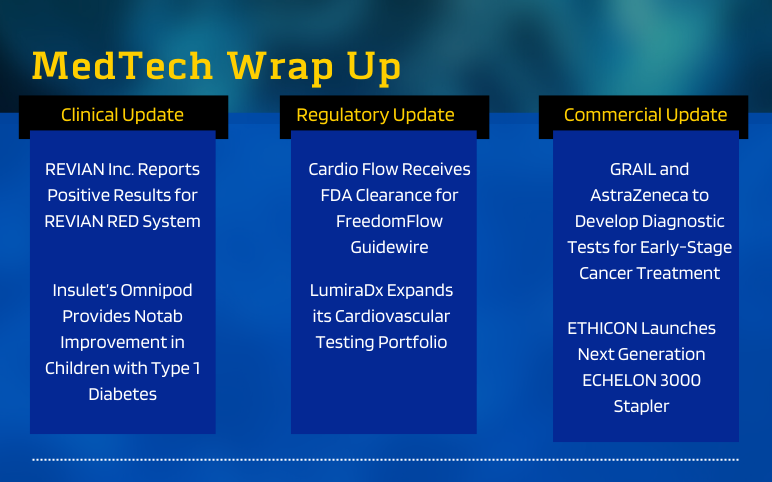
Positive Results Report REVIAN RED System as a Possible Treatment for Central Centrifugal Cicatricical Alopeciain African American Women On June 02, 2022, REVIAN Inc., an aesthetic medical technology company focused on stimulating the body's natural processes in order to rejuvenate hair and skin with light relea...
Find More
Diabetic Retinopathy (DR), a microvascular disease, is an eye condition in diabetic patients, affecting blood vessels in the retina and causing significant visual loss in working populations. The retina needs a constant supply of blood, which it receives through a network of tiny blood vessels. Constant high blood ...
Find More
Abbott Receives Regulatory Approval from US FDA for Alinity™ m STI Assay On May 04, 2022, the US Food and Drug Administration (FDA) granted the regulatory approval to Alinity™ m STI Assay developed by Abbott which is capable of simultaneously detecting and differentiating four common sexually trans...
Find More
MEDIT Announces Launch of i700 Intraoral Scanner On April 20, 2022, based on the success of i700 intraoral scanner, MEDIT Corp launched the all new i700 wireless intraoral scanner is light, fast, accurate, and now – wire-free. i700 wireless is a lightweight, wire-free device that offers a smooth and qui...
Find More
The American Society of Clinical Oncology (ASCO) is one of the largest and most respected conferences in the field of oncology. Held annually, this conference brings together researchers, physicians, and other healthcare professionals from around the world to discuss the latest advances in cancer research, diagnosis, and treatment.