Search by Categories
- Insights
- Rare Disease
- Oncology
- Cell and Gene Therapy
- Medical Devices
- Consulting
- News & Analysis
-
Therapeutic Areas

Our expertise in the industry
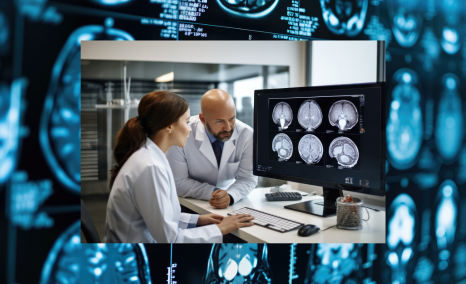
Glioma and glioblastoma are both types of brain tumors, but they differ significantly in their characteristics and prognosis. Glioma is a broad term that encompasses tumors originating from glial cells, which are supportive cells in the brain. These tumors can vary in grade and aggressiveness, with some growing slo...
Find More
FDA Granted Fast Track Designation to Oncternal Therapeutics’s ONCT-534 for the Treatment of Metastatic Castration-Resistant Prostate Cancer On Oct. 26, 2023, Oncternal Therapeutics, Inc. (Nasdaq: ONCT) announced that the U.S. Food and Drug Administration (FDA) has designated ONCT-534, its novel dual-acting andr...
Find More
FDA Approves First Gene Therapy for Severe Hemophilia A BioMarin Pharmaceutical Inc., a global biotechnology company dedicated to transforming lives through genetic discovery, announced that the US Food and Drug Administration (FDA) has approved ROCTAVIAN (valoctocogene roxaparvovec-rvox) gene therapy for the tr...
Find More
FDA Approves Jardiance for the Treatment of Type 2 Diabetes in Children 10 Years and Older Boehringer Ingelheim and Eli Lilly and Company announced that the FDA has approved Jardiance® (empagliflozin) 10 mg and 25 mg tablets to decrease blood sugar together with diet and exercise in children 10 years and older w...
Find More
FDA Approves ABRYSVO™, Pfizer’s Vaccine for the Prevention of Respiratory Syncytial Virus (RSV) in Older Adults Pfizer Inc. announced that the FDA has authorized ABRYSVO (Respiratory Syncytial Virus Vaccine), the company's bivalent RSV prefusion F (RSVpreF) vaccine, for the prevention of lower respiratory...
Find More
AstraZeneca’s Danicopan Shows Positive Results in Phase III Trial Danicopan, an oral Factor D inhibitor developed by AstraZeneca, was expected to fail a phase II trial in rare kidney disease in 2020, but a new readout could revive the drug. Danicopan (ALXN2040) has demonstrated efficacy as an adjunct treatment f...
Find More
Bexion Pharmaceuticals’ Early-Stage Cancer Drug, BXQ-350 Hailed as Game Changer For the Treatment of solid tumors and gliomas Bexion Pharmaceuticals’ investigational cancer treatment, BXQ-350, is receiving lots of mainstream media coverage as a potential game-changer. BXQ-350 is a formulation of a syntheticall...
Find More
Idera Pharmaceuticals Present Positive Phase I Data for their Drug candidate IMO-2125 Idera Pharmaceuticals announced final results from the dose-selection phase of an ongoing Phase 1/2 trial investigating IMO-2125 in combination with ipilimumab (Yervoy). The combination dose-selection phase included 18 patients, al...
Find More
The American Society of Clinical Oncology (ASCO) is one of the largest and most respected conferences in the field of oncology. Held annually, this conference brings together researchers, physicians, and other healthcare professionals from around the world to discuss the latest advances in cancer research, diagnosis, and treatment.