Search by Categories
- Insights
- Rare Disease
- Oncology
- Cell and Gene Therapy
- Medical Devices
- Consulting
- News & Analysis
-
Therapeutic Areas

Our expertise in the industry

Sarepta Therapeutics Announces Positive Vote from U.S. FDA Advisory Committee Meeting for SRP-9001 Gene Therapy Sarepta Therapeutics, Inc., a pioneer in precision genetic medicine for rare diseases, announced that the FDA's Cellular, Tissue, and Gene Therapies Advisory Committee (CTGTAC) voted 8 to 6 in favor of...
Find More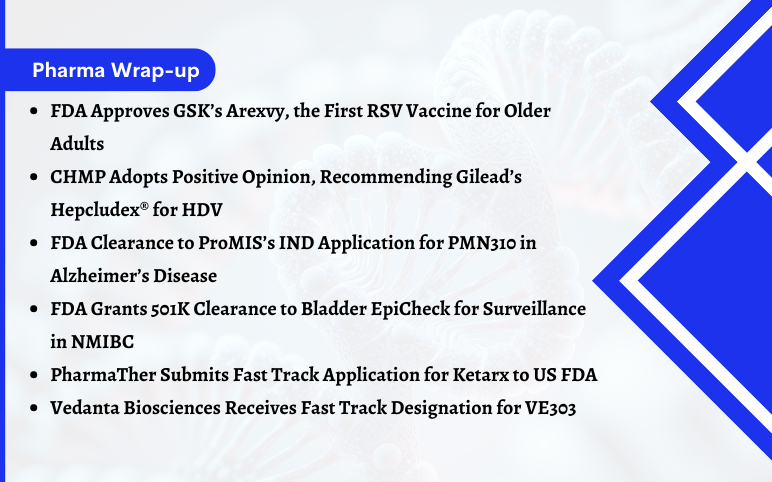
FDA Approves GSK’s Arexvy, the First RSV Vaccine for Older Adults GSK plc stated that the US Food and Drug Administration (FDA) has approved Arexvy (respiratory syncytial virus vaccine, adjuvanted) for the prevention of lower respiratory tract disease (LRTD) caused by a respiratory syncytial virus (RSV) in peopl...
Find More
FDA Grants Priority Review to Luspatercept for First-line Treatment of Anemia in Lower-risk MDS The FDA has granted priority review to a supplemental biologics license application (sBLA) seeking to expand the current indication of luspatercept-aamt (Reblozyl) to include treatment of anemia in patients with very ...
Find More
Janssen Marks First Approval Worldwide for AKEEGA® (Niraparib and Abiraterone Acetate Dual Action Tablet) The Janssen Pharmaceutical Companies of Johnson & Johnson announced that the European Commission (EC) had granted marketing authorization for AKEEGA® (niraparib and abiraterone acetate [AA]), in the form...
Find More
RegeneRx Biopharmaceuticals Enrolled First Patient in the Phase 3 Neurotrophic Keratitis Clinical Trial with RGN-259 in the US On April 12, 2023, RegeneRx Biopharmaceuticals, Inc. (OTCQB: RGRX) ("RegeneRx" or "Company") announced that the first patient of Phase 3 clinical trial (SEER-2) of RGN-259, a novel treat...
Find More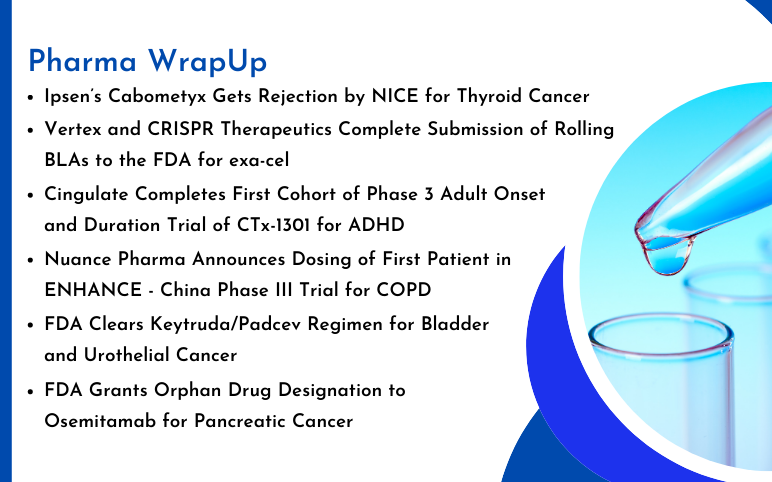
FDA Grants Orphan Drug Designation to Osemitamab for Pancreatic Cancer Transcenta Holding Limited has announced that the U.S. Food and Drug Administration (FDA) has awarded Orphan Drug Designation to Osemitamab (TST001), a highly potent humanized monoclonal antibody that enhances ADCC (antibody-dependent cell-me...
Find More
Novartis Announces the Positive Results of Phase III NATALEE trial Evaluating Kisqali Novartis announced positive topline results from an interim analysis of NATALEE, a Phase III trial evaluating Kisqali® (ribociclib) plus endocrine therapy (ET) in a broad population of patients with hormone receptor-positive/hu...
Find More
Viz.ai is First to Receive FDA 510(k) Clearance for AI Algorithm for Abdominal Aortic Aneurysm On March 21, 2023, Viz.ai, the leader in AI-powered disease detection and intelligent care coordination, announced it had received U.S. Food and Drug Administration (FDA) 510(k) clearance for its algorithm intended to ...
Find More
AbbVie Announces Positive Results of Study Evaluating SKYRIZI in Plaque Psoriasis Patients AbbVie announced new 52-week data from an open-label, a single-arm study demonstrating improved plaque psoriasis signs and symptoms in a difficult-to-treat patient population who received SKYRIZI® (risankizumab), an IL-23 ...
Find More
Incyte Halts Phase III Clinical Trial for Myelofibrosis Incyte announced the termination of Phase III LIMBER-304 trial after the results of a pre-planned interim analysis conducted by an independent data monitoring committee (IDMC) revealed that the study is unlikely to meet the primary endpoint in the int...
Find More
The American Society of Clinical Oncology (ASCO) is one of the largest and most respected conferences in the field of oncology. Held annually, this conference brings together researchers, physicians, and other healthcare professionals from around the world to discuss the latest advances in cancer research, diagnosis, and treatment.