Search by Categories
- Insights
- Rare Disease
- Oncology
- Cell and Gene Therapy
- Medical Devices
- Consulting
- News & Analysis
-
Therapeutic Areas

Our expertise in the industry

Sanofi to Acquire Provention Bio for USD 2.9 Billion Sanofi and Provention Bio, Inc., a publicly traded biopharmaceutical company based in the United States focused on intercepting and preventing immune-mediated diseases such as type 1 diabetes (T1D), have agreed to acquire Provention Bio, Inc. for $25.00 per sh...
Find More
Blood cancers have seen tremendous advancements in the past few years owing to the dedication of the researchers who are putting their endless efforts just to bring innovation into the lives of suffering patients. These experimentations and their upshots are always brought to us through various platforms, the Ameri...
Find More
Peripheral T-cell Lymphoma (PTCL) is a diverse disorder category that accounts for 10% to 15% of non-Hodgkin lymphomas. Most PTCL subtypes, including PTCL-NOS, AITL, ALCL, enteropathy-type T-cell lymphoma, and extranodal natural killer (NK) cell/T-cell lymphoma, are aggressive (fast-growing) lymphomas. PTCLs are...
Find More
Ipsen to Buy Biopharmaceutical Company Epizyme for USD 247 Million The restructuring of French manufacturer Ipsen has progressed with a takeover agreement for US competitor Epizyme and its cancer drug Tazverik in a deal for slightly less than USD 250 million. Ipsen is proposing USD 1.45 per share for Epizyme, va...
Find More
FDA Approves Yescarta as a First CAR T-cell Therapy for Initial Treatment of R/R Large B-cell Lymphoma Until now, existing CAR-T therapies have been reserved for patients with blood cancer who have tried multiple treatments. The FDA has approved Yescarta, a CD19-directed CAR-T therapy developed by Gilead Science...
Find More
Sandoz Launches generic Revlimid in 19 European Countries, Bringing a Flood of Competition to BMS' Megablockbuster Since Bristol Myers Squibb acquired Celgene and its megablockbuster Revlimid, the company has been bracing for the day when the multiple myeloma superstar would face generic competition. Sandoz, a s...
Find More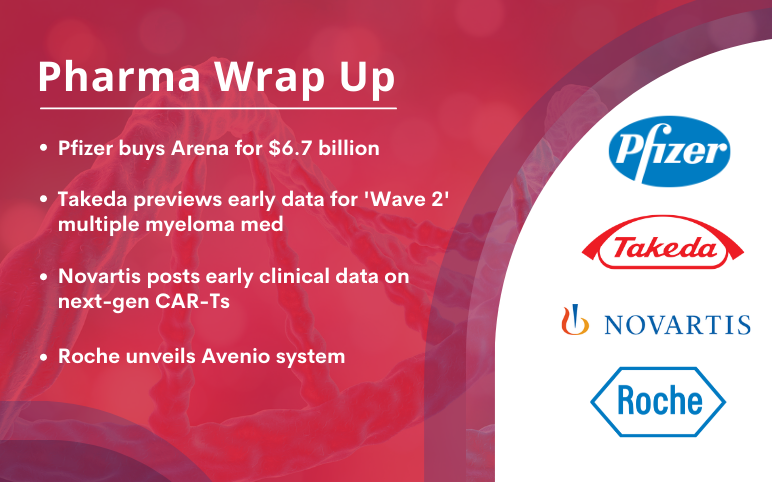
Pfizer acquires drugmaker Arena for USD 6.7 billion to address unmet needs Pfizer declared that it is acquiring Arena Pharmaceuticals, a drug company specializing in inflammation and immunology, for almost USD 7 billion. The pharma giant said it would finance the agreement, worth USD 6.7 billion, with cash on ha...
Find More
Progress is driven by innovation. When it comes to developing novel medications and therapeutic biological products, the FDA's Center for Drug Evaluation and Research (CDER) assists the pharmaceutical sector at every stage of the process. CDER offers scientific and regulatory assistance needed to bring innovative m...
Find More
Cidara Therapeutics Inks USD 780M Deal with J&J The share price of Cidara observed a sudden jump after the news of the collaboration of the company with J&J came. Cidara Therapeutics has announced a deal with Johnson & Johnson to develop and commercialize Cidara’s candidate CD388 to prevent and treat...
Find More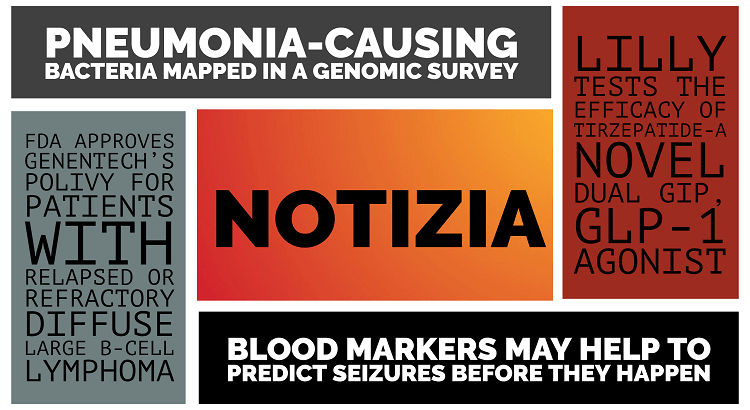
Lilly tests the efficacy of Tirzepatide-a novel dual GIP, GLP-1 agonist Eli Lilly is planning to test its diabetes drug Tirzepatide in a phase 2b Non-Alcoholic Steatohepatitis (NASH) trial. Tirzepatide, also known as LY3298176, has been excellent in reducing body weight and blood sugar in patients with poorly co...
Find More
The American Society of Clinical Oncology (ASCO) is one of the largest and most respected conferences in the field of oncology. Held annually, this conference brings together researchers, physicians, and other healthcare professionals from around the world to discuss the latest advances in cancer research, diagnosis, and treatment.