Search by Categories
- Insights
- Rare Disease
- Oncology
- Cell and Gene Therapy
- Medical Devices
- Consulting
- News & Analysis
-
Therapeutic Areas

Our expertise in the industry

Individuals suffering from food allergies now have access to a medication that can potentially avert serious consequences. This medication, which has been available for twenty years, has received approval from the FDA. Xolair (omalizumab) by Roche and Novartis is the first food allergy treatment sanctioned to dimin...
Find More
FDA Approves Xolair as First and Only Medicine for Children and Adults with One or More Food Allergies Roche has announced that the FDA has approved Xolair® (omalizumab) to mitigate allergic responses, such as anaphylaxis, that may arise from accidental exposure to various foods in both adult and pediatric patie...
Find More
China has become the first nation to approve Roche’s crovalimab, a paroxysmal nocturnal hemoglobinuria (PNH) treatment. Unlike AstraZeneca’s infused treatments Soliris and Ultomiris, crovalimab is administered subcutaneously. Developed by Roche’s subsidiary Chugai Pharmaceutical, crovalimab is a humanized complemen...
Find More
From January 8th to 11th, 2024, the 42nd Annual J.P. Morgan Healthcare Conference (JPM24) took center stage in San Francisco, CA, USA. Spanning four dynamic days, this conference saw the active participation of prominent figures from major pharmaceutical, biotechnology, Medtech, HealthTech entities, and emerging fa...
Find More
FDA Grants Priority Review to Merck’s Application for KEYTRUDA Plus Padcev for the First-Line Treatment of Patients With Locally Advanced or Metastatic Urothelial Cancer Merck, operating as MSD internationally, reported that the U.S. Food and Drug Administration (FDA) has prioritized the review of a supplementar...
Find More
Roche’s Vabysmo secures a new FDA nod for retinal vein occlusion treatment, intensifying the competition with Regeneron and Bayer’s Eylea in the eye medication market. This approval for Vabysmo in RVO adds another shared medical indication for the competing eye medications. Vabysmo’s successful FDA approval for RVO...
Find More
FDA Granted Fast Track Designation to Oncternal Therapeutics’s ONCT-534 for the Treatment of Metastatic Castration-Resistant Prostate Cancer On Oct. 26, 2023, Oncternal Therapeutics, Inc. (Nasdaq: ONCT) announced that the U.S. Food and Drug Administration (FDA) has designated ONCT-534, its novel dual-acting andr...
Find More
FDA Grants Breakthrough Therapy Designations to Trastuzumab Deruxtecan for HER2+ Solid Tumors, Including mCRC ENHERTU® (fam-trastuzumab deruxtecan-nxki) has been granted two additional Breakthrough Therapy Designations (BTDs) in the United States for the treatment of adult patients with unresectable or metastati...
Find More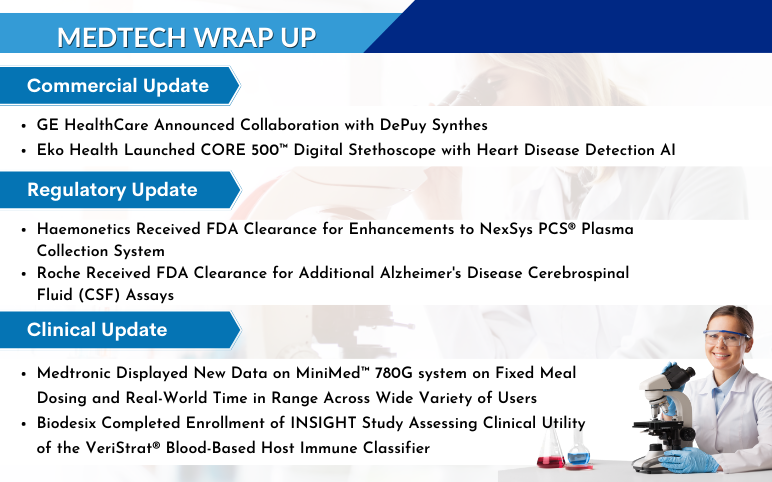
GE HealthCare Announced Collaboration with DePuy Synthes to Bring Advanced 3D Precision Imaging Innovation to Spine Practices in the United States On June 21, 2023, GE HealthCare entered into a distribution agreement with DePuy Synthes, the Orthopaedics division of Johnson & Johnson, to bring GE HealthCare’s...
Find More
GSK Announces Extension of FDA Review Period for Momelotinib After all, GSK will not hear from the FDA this month about its marketing application for momelotinib as a therapy for anemia in myelofibrosis patients. The pharmaceutical company announced that the US Food and Drug Administration has extended the drug'...
Find More
The American Society of Clinical Oncology (ASCO) is one of the largest and most respected conferences in the field of oncology. Held annually, this conference brings together researchers, physicians, and other healthcare professionals from around the world to discuss the latest advances in cancer research, diagnosis, and treatment.