Search by Categories
- Insights
- Rare Disease
- Oncology
- Cell and Gene Therapy
- Medical Devices
- Consulting
- News & Analysis
-
Therapeutic Areas

Our expertise in the industry
Juvenescence closes a USD 100 Million Series B round Juvenescence, a life sciences company focused on treating ageing problems, has successfully raised USD 100 Million in a Series B round. The investors who took part in the funding were Grok Ventures, the investment company of Mike Cannon-Brookes (Atlassian co-...
Find More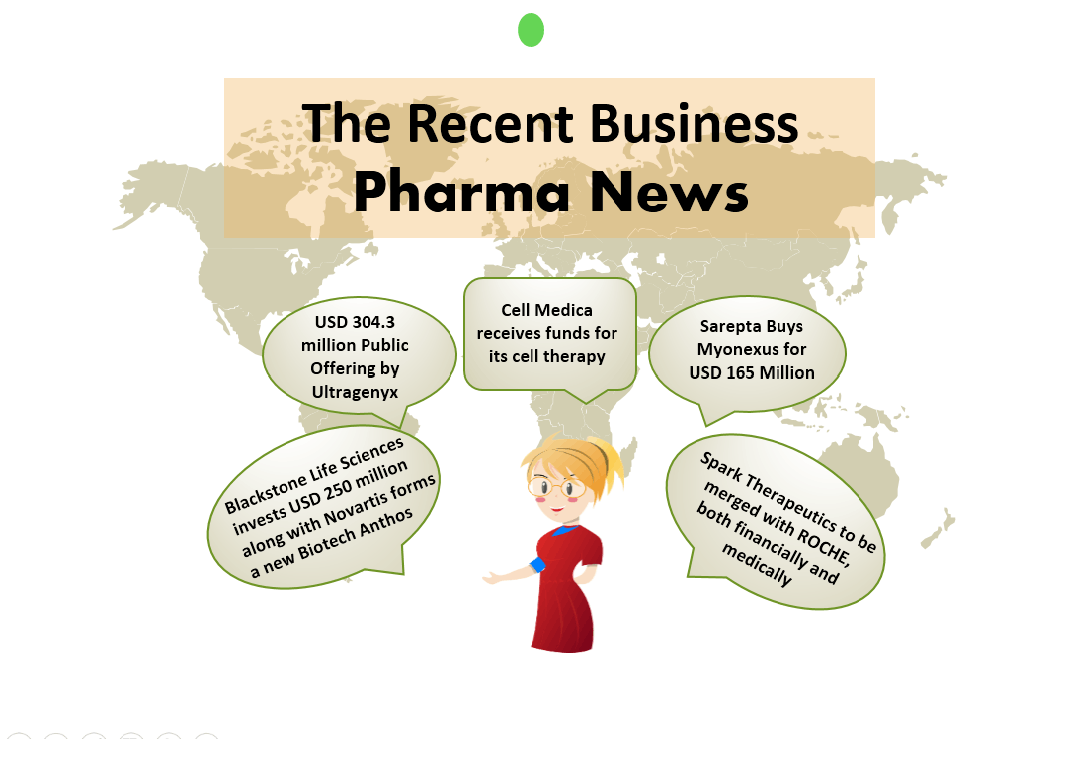
Cell Medica receives funds for its cell therapy A USD 8.7 million grant to Cell Medica has been given by Cancer Prevention and Research Institute of Texas (CPRIT) to speed up the clinical development of CMD-502 off-the-shelf chimeric antigen receptor-natural killer T cell (CAR-NKT) cell therapy. The program, su...
Find More
Sarepta builds in gene therapy with $30M Lacerta deal Sarepta has dampen its enthusiasm for an ongoing gene therapy candidate. Sarepta has made a tie-up with lacerta that will add 3 more programs to 8 already existing and are ready to expand its focus beyond muscular dystrophy. Sarepta has committed to pay Lacerta ...
Find More
Before approving an investigational drug, safety and effectivity needs to be proven. Something different happened with Sarepta’s Muscular dystrophy drug Eteplirsen, as its luck changed completely when the U.S. Food and Drug Administration on 19th September 2016 approved Sarepta Therapeutics' Exondys 51 (eteplirsen) ...
Find More
The American Society of Clinical Oncology (ASCO) is one of the largest and most respected conferences in the field of oncology. Held annually, this conference brings together researchers, physicians, and other healthcare professionals from around the world to discuss the latest advances in cancer research, diagnosis, and treatment.