
Cheers to new headways in Oncology: Are you all ready for ESMO 2021 Annual Meeting?
- Home
- esmo conference 2021
- new headways in oncology
Major Late-Breaking Abstracts
The ESMO 2021 annual meeting conference is all set to hit the road virtually from September 16th-21st, 2021, thereby again bringing an evolution in the field of oncology. Oncologists in these medical meetings, with their active discussions involving question and answers (QnA) from their live audience, brings such enthusiasm not only among the viewers but also amongst the patients. On this note, DelveInsight has listed down a few of the major late-breaking abstracts (and summarized only a few) that might bring a change in the upcoming future with an intent to transform the lives of the patients.
DESTINY-Breast03 in the Spotlight
DESTINY-Breast03 by AstraZeneca is a global head-to-head, randomized, open-label, registrational phase III trial evaluating the safety and efficacy of Enhertu versus Trastuzumab Emtansine in patients with HER2-positive unresectable and/or metastatic breast cancer previously treated with trastuzumab and a taxane. The company recently, in its press release, stated that the Independent Data Monitoring Committee (IDMC) concluded that DESTINY-Breast03 met the primary endpoint of progression-free survival (PFS), showing a highly statistically significant and clinically meaningful improvement for the patients. The full results from the study will be published under the late-breaking abstracts, which are expected to show good clinical benefits in such patients.
Much awaited results from the KEYNOTE-826 study
KEYNOTE-826, by Merck, is a randomized, triple-blind, phase III trial evaluating Keytruda in combination with platinum-based chemotherapy plus carboplatin with or without bevacizumab compared with placebo in combination with the same platinum-based chemotherapy regimens with or without bevacizumab for the first-line treatment of adult patients with persistent, recurrent or metastatic cervical cancer. Recently in June 2021, the company, in its press release, stated that the trial met its primary endpoints of overall survival (OS) and progression-free survival (PFS). It is also the confirmatory trial for the current accelerated approval for KEYTRUDA in cervical cancer for the second-line treatment of patients with recurrent or metastatic cervical cancer with disease progression on or after chemotherapy whose tumors express PD-L1 (Combined Positive Score [CPS] ≥1) as determined by an FDA-approved test.
Everybody is eyeing Adagrasib from KRYSTAL-1 Study
It has taken decades of research to finally target KRAS mutation, and players such as Amgen, Mirati Therapeutics, Eli Lilly, Boehringer Ingelheim, Cardiff Oncology, and Revolution Medicines, along with Merck, are leading the development. KRYSTAL-1 trial by Mirati is a phase I/II trial evaluating the clinical activity of MRTX849 (adagrasib) in patients with advanced solid tumors that have a KRAS G12C mutation. This year the company has planned to publish the data readout from one of its cohorts evaluating adagrasib (MRTX849) as monotherapy or combined with cetuximab in second-line patients with a KRASG12C mutation who have received three or more lines of therapy.
The key abstracts have been summarized in the table below whereas the most promising and hyped abstracts to be published in the conference will be described in the upcoming posts of DelveInsight:
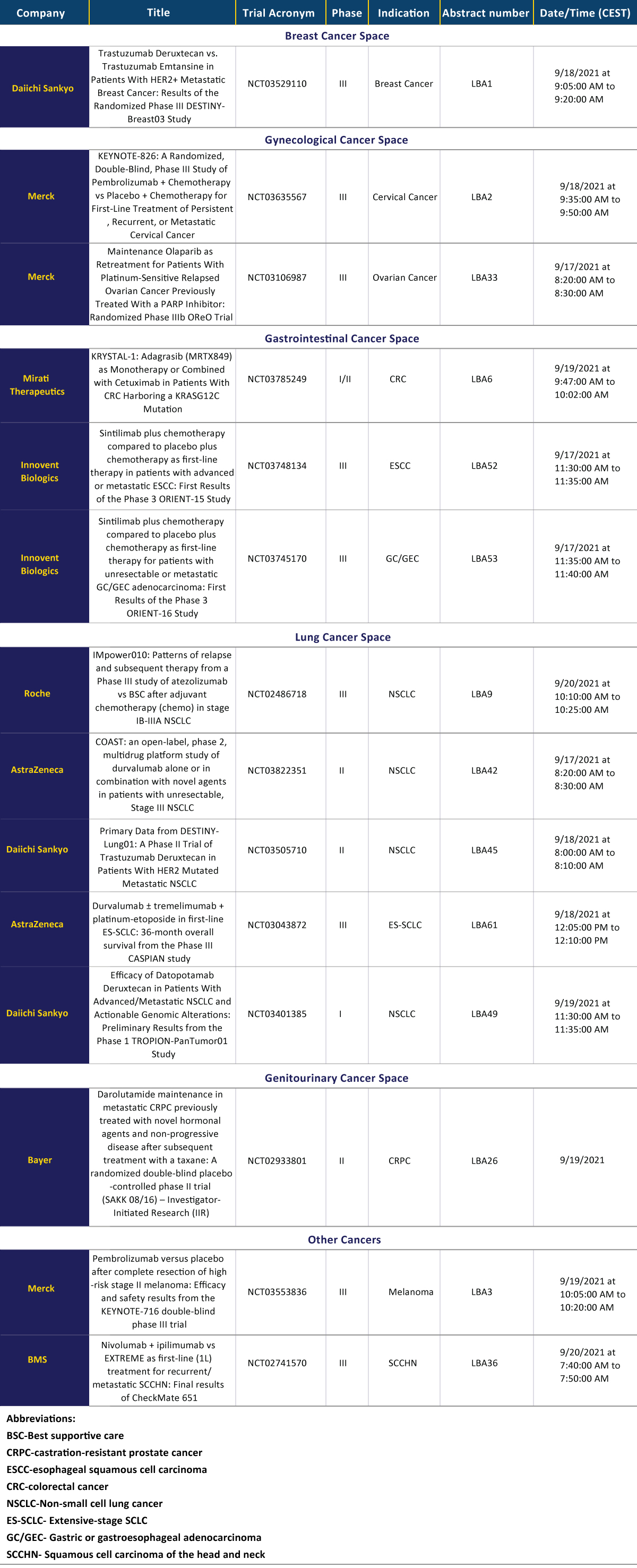
Executive Summary
DelveInsight has listed down a few of the major late-breaking abstracts (and summarized only a few) that might bring a change in the upcoming future with an intent to transform the lives of the patients.