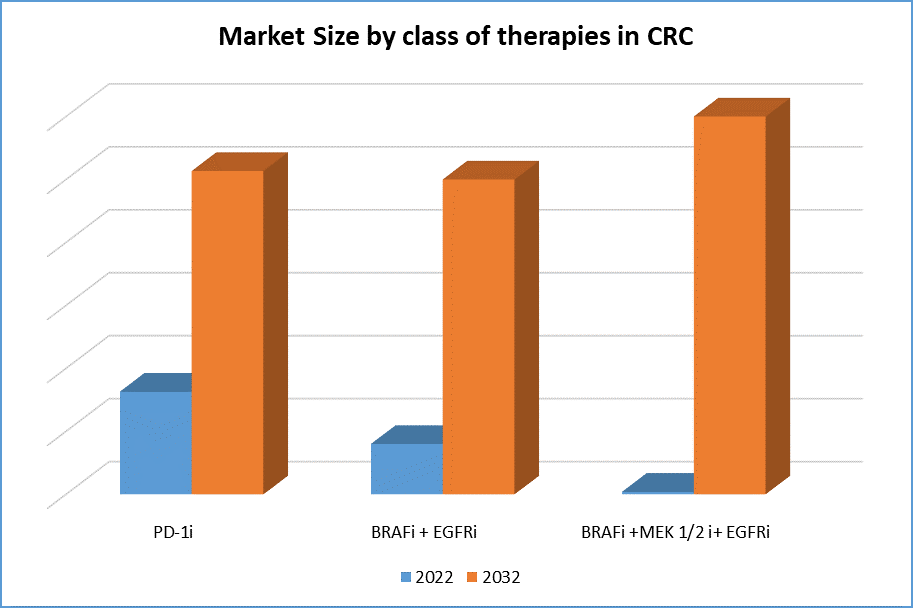
Already established combination in refractory mCRC patients to show results in the front-line patients to capture market share
- Home
- esmo conference 2022
- encorafenib plus cetuximab
To evaluate the safety and efficacy of encorafenib plus cetuximab with or without chemotherapy as compared to chemo alone
BREAKWATER Phase III is currently enrolling patients with treatment-naïve, BRAF V600E-mutated mCRC to evaluate the efficacy and safety of encorafenib plus cetuximab with or without chemotherapy as compared to chemo alone. Readouts from an interim analysis of this pivotal trial are presented at the upcoming ESMO conference. Last year, in September 2021, US FDA approved this combination for use in adult patients with mCRC and a BRAF V600E mutation following prior therapy, based on findings from the Phase III BEACON CRC trial (NCT02928224). This was the first approval of targeted therapies and is now the standard for BRAF-mutated CRC in a second-line setting. Patients with mCRC whose tumors harbor BRAF V600E mutations have limited options available to them in the frontline setting. To date, these patients receive cytotoxic chemotherapy with or without anti-VEGF or anti-EGFR therapies. The BREAKWATER trial aims to address this unmet need by evaluating encorafenib plus cetuximab with or without chemotherapy as a potential up-front treatment for this population. This BREAKWATER study seeks to answer whether a higher response rate will be observed in the frontline setting. Additionally, the trial will explore whether there is a similar synergy between cytotoxic and targeted therapies in this patient population as has been observed in other settings in CRC. Giving potential coverage across all lines of therapies results from this BREAKWATER study will be crucial for Pfizer and help gain a stronghold in the CRC landscape.
Executive Summary
Anticipated encouraging safety and efficacy results of an already approved combination of encorafenib and cetuximab (in a 2L setting) in CRC patients who have not received any prior therapy and are naïve to treatment.