acute lymphoblastic leukemia all market
DelveInsight's " Acute Lymphoblastic Leukemia Market Insights, Epidemiology, and Market Forecast-2030 " report delivers an in-depth understanding of the Acute Lymphoblastic Leukemia (ALL), historical and forecasted epidemiology as well as the Acute Lymphoblastic Leukemia market trends in the United States, EU5 (Germany, Spain, Italy, France, and United Kingdom) and Japan.
The Acute Lymphoblastic Leukemia market report provides current treatment practices, Acute Lymphoblastic Leukemia emerging drugs, Acute Lymphoblastic Leukemia market share of the individual therapies, current and forecasted Acute Lymphoblastic Leukemia (ALL) market Size from 2017 to 2030 segmented by seven major markets. The Report also covers current Acute Lymphoblastic Leukemia treatment practice/algorithm, Acute Lymphoblastic Leukemia market drivers, Acute Lymphoblastic Leukemia market barriers and unmet medical needs to curate best of the opportunities and assesses the underlying potential of the market.
Geography Covered
- The United States
- EU5 (Germany, France, Italy, Spain, and the United Kingdom)
- Japan
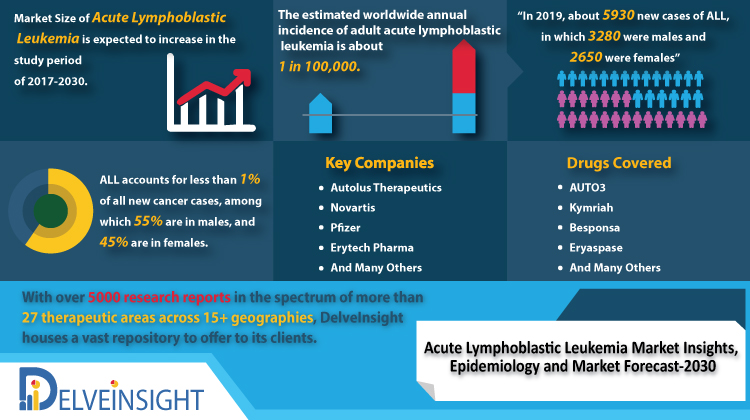
Study Period: 2017-2030
Acute Lymphoblastic Leukemia Disease Understanding and Treatment Algorithm
Leukemia is a term given to a group of cancers that develop in the blood and bone marrow. It originates in developing blood cells that have undergone a malignant change, which means they multiply in an uncontrolled manner, leaving them unformed and inoperative.
Leukemia can be either acute or chronic. In chronic leukemia, there is an accumulation of mature but abnormal white blood cells that have undergone a malignant change when developing from a blast cell. It progresses more slowly than acute leukemia and may not require treatment for a long time after it is diagnosed.
On the other hand, with acute leukemia, the diseased bone marrow produces an excessive number of abnormal blast cells, called leukemic cells. These cells accumulate in the bone marrow interfering with the production of normal blood cells. Acute leukemia develops and progresses quickly, and therefore, needs to be treated as soon as it is detected.
Typical forms of acute leukemia include acute myeloid leukemia (AML), acute lymphocytic leukemia (ALL), and acute promyelocytic leukemia (APML).
Acute lymphocytic leukemia (ALL), also known as acute lymphoblastic leukemia, is a type of cancer that affects the blood and bone marrow. It starts from young white blood cells called lymphocytes in the bone marrow; mainly characterized by an overproduction of immature white blood cells, called lymphoblasts or leukemic blasts. Because the bone marrow is unable to make adequate numbers of red cells, normal white cells, and platelets, people with ALL become more susceptible to anemia, recurrent infections, and to bruising and bleeding easily. The blast cells can then spill out of the bone marrow into the bloodstream and accumulate in various organs including the lymph nodes or glands, spleen, liver, and central nervous system (brain and spinal cord).
ALL is mainly classified into B-cell and T-cell ALL. ALL can occur at any age but is more common in young children (0–14 years) and it develops quickly, around 54% of ALL cases in US diagnosed among people aged <20 years. Among children, B-cell lineage ALL constitutes approximately 88% of cases. Among adults, B-cell lineage represents around 75% of cases.
Acute Lymphocytic Leukemia Diagnosis
Certain signs and symptoms can suggest that a person might have ALL, but tests are needed to confirm the diagnosis. During the physical exam, the doctor usually focus on any enlarged lymph nodes, areas of bleeding or bruising, or possible signs of infection. The eyes, mouth, and skin will be looked at carefully, and a thorough nervous system exam may be done. The patient’s abdomen will be checked for spleen or liver enlargement.
If there is reason to think low levels of blood cells might be causing symptoms (anemia, infections, bleeding or bruising, etc.), the doctor will most likely order blood tests to check blood cell counts. The patient might also be referred to a hematologist doctor who specializes in diseases of the blood, including leukemia.
The diagnosis of ALL is mainly done by Blood test (complete blood count (CBC) and peripheral blood smear, blood chemistry tests, blood coagulation tests), Bone marrow test (bone marrow aspiration and biopsy), Lab tests (routine exams with a microscope, cytochemistry tests, flow cytometry and immunohistochemistry), Chromosome tests (fluorescent in situ hybridization (FISH), polymerase chain reaction (PCR)), Imaging tests (computerized Tomography (CT) scan, magnetic resonance imaging (MRI) Scan, and staging).
Acute Lymphocytic Leukemia Treatment
ALL is a malignant clonal disease that usually develops when a lymphoid progenitor cell turns into genetically altered through somatic changes and goes through uncontrolled proliferation. This progression of clonal expansion further leads to ALL. However, common treatment of ALL divided into distinct phases such as Induction therapy, Consolidation therapy, Maintenance therapy, and Preventive treatment to the spinal cord, among others.
- Induction Therapy - The main purpose of the first phase of Acute Lymphoblastic Leukemia treatment is to kill most of the leukemia cells in the bone marrow and blood also to restore normal blood cell production.
- Consolidation Therapy - Consolidation therapy is also known as post-remission therapy. The main purpose of this therapy is to completely wipe out remaining leukemia in the body, such as in the brain or spinal cord. Consolidation therapy is also known as post-remission therapy.
- Maintenance Therapy - This is known as the third phase of Acute Lymphoblastic Leukemia treatment, which prevents leukemia cells from regrowth. However, the treatment used in this stage is often given at much lesser doses for a long period, often years.
- Preventive treatment to the spinal cord - In this phase of therapy, a patient suffering from ALL may receive additional treatment from killing leukemia cells which are located in the central nervous system. Also, in this type of treatment phase chemotherapy drugs are often injected directly into the fluid that covers the spinal cord.
The therapies that are approved for the Acute Lymphoblastic Leukemia treatment are Blincyto (blinatumomab/MT 103), Kymriah {CTL019 (tisagenlecleucel)}, Besponsa (inotuzumab ozogamicin), Iclusig (Ponatinib), among others.
Acute Lymphoblastic Leukemia Epidemiology
The Acute Lymphoblastic Leukemia epidemiology division provide insights about historical and current Acute Lymphoblastic Leukemia patient pool and forecasted trend for every seven major countries. It helps to recognize the causes of current and forecasted trends by exploring numerous studies and views of key opinion leaders. This part of the DelveInsight report also provides the diagnosed patient pool and their trends along with assumptions undertaken.
Key Findings
In the year 2017, the 7MM total incident case of ALL was 10,341 cases which are expected to grow during the study period, i.e., 2017–2030.
The disease epidemiology covered in the report provides historical as well as forecasted ALL epidemiology [segmented as Total Incident Cases of Leukemia, Total Incident Cases of ALL, Gender-specific cases of ALL, Diagnosed cases of ALL by Age Distribution, Subtype-specific cases of ALL, Genetic mutation-specific cases of ALL, and Total Treated Cases of ALL] scenario of ALL in the 7MM covering United States, EU5 countries (Germany, France, Italy, Spain, and United Kingdom), and Japan from 2017 to 2030.
Country Wise- Acute Lymphoblastic Leukemia (ALL) Epidemiology
Estimates show that the highest cases of ALL in the 7MM were in the United States, followed by Germany, Japan, France, the United Kingdom, Italy, and Spain in 2017.
- In the United States, the total number of Acute Lymphoblastic Leukemia incident cases were 5,816 cases in the year 2017 which are expected to grow during the study period, i.e., 2017–2030.
- In the year 2017, the total Acute Lymphoblastic Leukemia incident cases were 3,652 cases in EU-5 which are expected to grow during the study period, i.e., 2017–2030.
- In Japan, the total number of Acute Lymphoblastic Leukemia incident cases were 872 cases in the year 2017 which are expected to grow during the study period, i.e., 2017–2030.
Acute Lymphoblastic Leukemia Recent Developments
- On October 29, 2024, Shorla Oncology, a specialty pharmaceutical company based in the U.S. and Ireland, announced that the FDA has expanded the approval of JYLAMVO™ (methotrexate) to treat pediatric patients with acute lymphoblastic leukemia (ALL) and polyarticular juvenile idiopathic arthritis (pJIA). With this approval, JYLAMVO is now the only oral liquid methotrexate on the market approved for both adult and pediatric use.
Acute Lymphoblastic Leukemia Drug Chapters
Drug chapter segment of the Acute Lymphoblastic Leukemia report encloses the detailed analysis of Acute Lymphoblastic Leukemia marketed drugs and late stage (Phase-III and Phase-II) pipeline drugs. It also helps to understand the Acute Lymphoblastic Leukemia (ALL) clinical trial details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug and the latest news and press releases.
Acute Lymphoblastic Leukemia Marketed Drugs
Blincyto/blinatumomab/MT 103 (Amgen)
Blincyto is a bispecific CD19-directed CD3 T-cell engager (BiTE) immunotherapy that binds to CD19 expressed on the surface of cells of B-lineage origin and CD3 expressed on the surface of T-cells. Blinatumomab possesses two antigen-recognition sites, one for the CD3 complex, a group of T-cell surface glycoproteins that complex with the T-cell receptor (TCR), and one for CD19, a tumor-associated antigen (TAA) overexpressed on the surface of B-cells. This bispecific monoclonal antibody brings CD19-expressing tumor B-cells and cytotoxic T lymphocytes (CTLs) and helper T lymphocytes (HTLs) together, which may result in the CTL- and HTL-mediated cell death of CD19-expressing B-lymphocytes.
In July 2014, the US FDA granted Breakthrough Therapy Designation to Blincyto for adults with Philadelphia-negative (Ph-) relapsed/refractory B-precursor ALL. Before this, in May 2008, the US FDA granted orphan drug designation to blinatumomab for the treatment of ALL.
Furthermore, in July 2009, the European Commission granted orphan drug designation to Micromet AG, Germany, for blinatumomab for the treatment of acute lymphoblastic leukemia.
Asparlas/calaspargase pegol-mknl (Servier Pharmaceuticals)
Asparlas (calaspargase pegol-mknl) is an intravenous formulation containing E.coli-derived L-asparaginase II conjugated with succinimidyl carbonate monomethoxypolyethylene glycol (SC-PEG), with potential antineoplastic activity. L-asparaginase hydrolyzes L-asparagine to L-aspartic acid and ammonia, thus depleting cells of asparagine. Asparagine depletion blocks protein synthesis and tumor cell proliferation, especially in the G1 phase of the cell cycle and ultimately induces tumor cell death. Asparagine is critical to protein synthesis in acute lymphoblastic leukemia (ALL) cells which, unlike normal cells, cannot synthesize this amino acid due to the absence of the enzyme asparagine synthase.
In April 2018, Servier entered into a definitive agreement with the Shire, a leading global biotechnology company focused on rare diseases, to acquire its Oncology business for USD 2.4 Billion. The acquisition allows Servier to establish an immediate and direct commercial presence in the United States, the world’s leading biopharmaceuticals market.
Kymriah/tisagenlecleucel (Novartis Pharmaceuticals)
Kymriah (tisagenlecleucel, formerly CTL019) suspension for intravenous infusion is a CD19-directed genetically modified autologous chimeric antigen receptor T-cell (CAR-T) therapy. It is approved in the US, the EU, Japan, and other countries for the treatment of:
- Patients up to 25 years with B-cell acute lymphoblastic leukemia that is refractory or in second or later relapse
- Adults with relapsed or refractory diffuse large B-cell lymphoma after two or more lines of systemic therapy
In January 2014, the US FDA granted Orphan drug designation to Kymriah for the treatment of ALL. Likewise in April 2014, Orphan drug designation was granted by the European Commission to Novartis for the treatment of B-lymphoblastic leukemia/lymphoma.
In addition to this, the US FDA granted Kymriah a breakthrough therapy designation for relapsed or refractory B-cell ALL.
Besponsa/inotuzumabozogamicin (Pfizer)
Besponsa is an antibody-drug conjugate (ADC) composed of a monoclonal antibody (mAb) targeting CD22, a cell surface antigen expressed on cancer cells in almost all B-ALL patients, linked to a cytotoxic agent. It is used for the treatment of adults with relapsed or refractory B-cell precursor ALL. When Besponsa binds to the CD22 antigen on B-cells, it is internalized into the cell, where the cytotoxic agent calicheamicin is released causing cell death. Besponsa originated from a collaboration between Pfizer and Cell tech, now UCB.
In March 2013, the US FDA also granted Inotuzumab ozogamicin with the Orphan Designation Status for the treatment of B-cell ALL. Later, in June 2013, the orphan designation was granted by the European Commission to Pfizer for inotuzumab ozogamicin for the treatment of B-cell ALL.
In October 2015, Inotuzumab ozogamicin was granted with Breakthrough Therapy designation from the US FDA for ALL.
Iclusig/Ponatinib (Takeda/Ariad Pharmaceuticals)
Iclusig is an orally administered kinase inhibitor whose primary target is BCR-ABL, an abnormal tyrosine kinase that is expressed in chronic myeloid leukemia (CML) and Philadelphia-chromosome positive acute lymphoblastic leukemia (Ph+ ALL). Iclusig was designed using ARIAD’s computational and structure-based drug-design platform specifically to inhibit the activity of BCR-ABL. It targets not only native BCR-ABL but also its isoforms that carry mutations that confer resistance to treatment, including the T315I mutation, which has been associated with resistance to other approved TKIs.
It is used in the treatment of the following:
- Treatment of adult patients with chronic phase, accelerated phase, or blast phase chronic myeloid leukemia (CML) or Ph+ ALL for whom no other tyrosine kinase inhibitor (TKI) therapy is indicated.
- Treatment of adult patients with T315I-positive CML (chronic phase, accelerated phase, or blast phase) or T315I-positive Ph+ ALL
Note: Detailed Current therapies assessment will be provided in the full report of ALL
Acute Lymphoblastic Leukemia (ALL) Emerging Drugs
PBCAR0191 (Precision BioSciences/Servier)
Precision BioSciences is investigating their first allogeneic CAR T in Phase I/II clinical trial for relapsed/refractory cases of B-cell ALL and NHL. This product is under investigation in collaboration with Servier. PBCAR0191 is an allogeneic CAR T cell therapy targeting the well-validated tumor target CD19 and is being developed for ALL, and non-hodgkin lymphoma, or NHL. Also, CD19 is a protein that is expressed on the surface of B-cells.
This product is based on the donor-derived T-cells modified using the ARCUS genome editing technology. PBCAR0191 recognizes the well-characterized tumor cell surface protein CD19, an important and validated target in several B-cell cancers. It is designed to avoid graft-versus-host disease, or GvHD, a significant complication associated with donor-derived, cell-based therapies.
AUTO1 (Autolus Limited)
Autolus Limited is also investigating its lead CAR T-cell therapy candidate in pediatric and young adult patients with ALL. It is a CD19 CAR T-cell investigational therapy designed to overcome the limitations in safety—while maintaining similar levels of efficacy—compared to current CD19 CAR T cell therapies. Designed to have a fast target binding off-rate to minimize excessive activation of the programmed T cells, AUTO1 may reduce toxicity and be less prone to T-cell exhaustion, which could enhance persistence and improve the T-cells’ abilities to engage in serial killing of target cancer cells. AUTO1 is Autolus’ most advanced program and recently entered a pivotal study in adult ALL and is also being evaluated in a Phase I study in pediatric ALL.
In April 2020, the US FDA has accepted the IND application for AUTO1, its lead CAR T product candidate for the treatment of adults with ALL. The active IND allows initiation of the US sites in the company’s first pivotal study, AUTO1-AL1. In November 2019, the US FDA granted AUTO1 orphan drug designation for the treatment of ALL patients.
KTE-X19 (Gilead Sciences)
KTE-X19 is an investigational, autologous, anti-CD19 CAR T cell therapy. KTE-X19 uses the XLP manufacturing process that includes T-cell selection and lymphocyte enrichment. Lymphocyte enrichment is a necessary step in certain B-cell malignancies in which circulating lymphoblasts are a common feature. It is a preparation of autologous peripheral blood T lymphocytes (PBTL) that have been transduced with a retroviral vector expressing a chimeric antigen receptor (CAR) consisting of an anti-CD19 single chain variable fragment (scFv) coupled to the costimulatory signaling domain CD28 and the zeta chain of the T-cell receptor (TCR)/CD3 complex (CD3 zeta), with potential immune stimulating and antineoplastic activities. Upon intravenous infusion and re-introduction of autologous anti-CD19 CAR-CD28 T cells KTE-X19 into the patient, these cells bind to and induce selective toxicity in CD19-expressing tumor cells.
UCART19 (Servier/Allogene)
UCART19 is a first-in-class allogeneic CAR T cell product candidate for the treatment of pediatric and adult patients with R/R CD19 positive B-cell ALL. Servier is the sponsor of the UCART19 clinical trials and is also responsible for manufacturing UCART19. This therapy is being jointly developed under a clinical development collaboration between Servier and Allogene based on an exclusive license granted by Cellectis to Servier. UCART19 utilizes TALEN gene-editing technology pioneered and owned by Cellectis.
UCART19 is manufactured to express a CAR that is designed to target CD19 and gene-edited to lack TCRα and CD52 to minimize the risk of GvHD and enable a window of persistence in the patient. In addition, UCART19 cells are engineered to express a small protein on the cell surface called RQR8, which consists of two rituximab recognition domains. This allows for recognition and elimination of cells if silencing of CAR activity is desired.
In February 2020, Cellectis granted additional rights to Servier to develop and commercialize all next-generation gene-edited allogeneic CAR T-cell products targeting CD19, including ALLO-501A.
Lisocabtagene Maraleucel/JCAR017 (Bristol-Myers Squibb)
Lisocabtagene Maraleucel (JCAR017), also known as Liso-cel, is under development by Bristol-Myers Squibb. It is an investigational CAR T-cell therapy designed to target CD19, which is a surface glycoprotein expressed during normal B-cell development and maintained following malignant transformation of B cells. Liso-cel CAR T cells aim to target CD19 expressing cells through a CAR construct that includes an anti-CD19 single-chain variable fragment (scFv) targeting domain for antigen specificity, a transmembrane domain, a 4-1BB co-stimulatory domain hypothesized to increase T-cell proliferation and persistence, and a CD3-zeta T-cell activation domain. The defined composition of CD4+ and CD8+ CAR T cells in liso-cel may limit product variability; however, the clinical significance of defined composition is unknown.
In September 2016, the US FDA granted orphan drug designation to JCAR017 for the treatment of ALL.
Venetoclax/Venclexta/ABT199/RG7601 (AbbVie and Roche)
Venetoclax (Venclexta, Venclyxto) is an oral B-cell lymphoma-2 (BCL-2) inhibitor developed by AbbVie and Genentech. It is used for the treatment of adult patients with Chronic Lymphocytic Leukemia (CLL) or Small Lymphocytic Leukemia (SLL) and in combination with azacitidine or decitabine or low-dose cytarabine for the treatment of newly-diagnosed acute myeloid leukemia (AML) in adults who are aged 75 or older, or who have comorbidities that preclude the use of intensive induction chemotherapy.
Venetoclax helps restore the process of apoptosis by binding directly to the BCL-2 protein, displacing proapoptotic proteins like BIM, triggering mitochondrial outer membrane permeabilization, and the activation of caspases. In nonclinical studies, venetoclax has demonstrated cytotoxic activity in tumor cells that overexpress BCL-2.
JZP-458/PF743/recombinant Erwinia asparaginase (Jazz Pharmaceuticals)
Jazz Pharmaceuticals is investigating JZP-458 for the treatment for pediatric and adult patients with ALL who are hypersensitive to E. coli-derived asparaginases. JZP-458 is a recombinant of Erwinia asparaginase which uses a novel Pseudomonas fluorescens manifestation platform. This product is in development by using the Pfenex’s Expression technology under their agreement with Jazz Pharmaceuticals. Pfenex granted worldwide rights to develop and commercialize multiple early-stage hematology product candidates, including a recombinant Erwinia asparaginase JZP-458 to Jazz pharmaceuticals.
In October 2019, the US Food and Drug Administration granted Fast Track Designation for JZP-458/PF743 for the treatment of ALL.
Daratumumab (Janssen Research & Development)
Daratumumab is a human IgG1k monoclonal antibody that binds with high affinity to the CD38 molecule, which is highly expressed on the surface of multiple myeloma cells. Daratumumab is being developed by Janssen Biotech under an exclusive worldwide license to develop, manufacture and commercialize daratumumab from Genmab. This drug has been already approved by the US FDA to treat multiple myeloma with the brand name Darzalex. Janssen Research & Development had initiated an open-label, multicenter, phase II study evaluating the efficacy and safety of Daratumumab in pediatric and young adult subjects ≥1 and ≤30 years with relapsed/refractory precursor B-cell or T-cell ALL or lymphoblastic lymphoma.
In July 2012, Genmab entered into a collaboration with Janssen Biotech and its affiliates (Janssen) to create and develop bispecific antibodies using its DuoBody technology platform. Genmab created panels of bispecific antibodies to multiple disease target combinations identified by Janssen, who will in turn fully fund research at Genmab.
Imbruvica/Ibrutinib {Pharmacyclics (an AbbVie Company)}
Ibrutinib is an oral small-molecule inhibitor of type of enzyme, called a protein kinase that controls the rate at which certain cells multiply. In particular, ibrutinib has been shown to bind to covalently, and ultimately inhibit, the Bruton’s tyrosine kinase (BTK). BTK plays a primary role in signaling healthy B cells to survive, mature, proliferate and release antibodies.
Since its launch in 2013, Imbruvica had received 11 FDA approvals across six disease areas: chronic lymphocytic leukemia (CLL) with or without 17p deletion (del17p); small lymphocytic lymphoma (SLL) with or without del17p; Waldenström's macroglobulinemia (WM); previously-treated patients with mantle cell lymphoma (MCL); previously-treated patients with marginal zone lymphoma (MZL) who require systemic therapy and have received at least one prior anti-CD20-based therapy and previously-treated patients with chronic graft-versus-host disease (cGVHD) after the failure of one or more lines of systemic therapy.
Note: Detailed emerging therapies assessment will be provided in the final report.
Acute Lymphoblastic Leukemia Market Outlook
Chemotherapy is often complex and intense, particularly in the initial months of treatment for Acute Lymphoblastic Leukemia. The most common Acute Lymphoblastic Leukemia treatment regimens use a combination of more than one anticancer drug. It is broken down into three phases: induction phase, consolidation (or intensification) phase, and maintenance phase. Induction is the first phase of chemotherapy, and the goal of this phase is to induce a remission. In this phase, numerous Acute Lymphoblastic Leukemia drugs are usually being used depending on the patient’s age, the specific features of leukemia, and the overall health of the patient. Induction regimens for ALL generally use a combination of drugs that include vincristine; anthracyclines (daunorubicin, doxorubicin); and corticosteroids (prednisone, dexamethasone) administered either with or without asparaginase and/or cyclophosphamide. Even after the complete remission, some leukemia cells still remain in the body. The presence of these cells is referred to as “minimal residual disease (MRD).” Patients who have MRD, are at increased risk of disease relapse. After a patient achieves a complete remission, postremission therapy is given to kill every remaining leukemia cell in the body.
Oftentimes when residual leukemia cells remain after remission, so the optimal treatment for Acute Lymphoblastic Leukemia patients requires additional intensive postremission therapy. The second phase of chemotherapy is called consolidation therapy. The combination of drugs and the duration of therapy for consolidation regimens vary but can consist of combinations of drugs similar to those drugs used during the induction phase. Some drugs which are used in this phase are High-dose methotrexate, Cytarabine, Vincristine, 6-mercaptopurine, Blincyto, Besponsa, Cyclophosphamide, Asparaginase, and Corticosteroids (prednisone, and dexamethasone). The third phase of ALL treatment is called “maintenance phase.” The goal of maintenance therapy is to prevent disease relapse after induction and consolidation therapy. Most maintenance regimens include 6-mercaptopurine, Methotrexate, Vincristine, Corticosteroids, and Intrathecal chemotherapy.
At present several pharmaceutical companies are working for the development of novel approach to treat this condition. Key players like KTE-X19 (Gilead Sciences), UCART19 (Servier/Allogene), Lisocabtagene Maraleucel/JCAR017 (Bristol-Myers Squibb), Venetoclax/Venclexta/ABT199/RG7601 (AbbVie and Roche), JZP-458/PF743/recombinant Erwinia asparaginase (Jazz Pharmaceuticals), Daratumumab (Janssen Research & Development), Imbruvica/Ibrutinib {Pharmacyclics (an AbbVie Company)}, AUTO1 (Autolus Limited), PBCAR0191 (Precision BioSciences/Servier), and others.
Key Findings
The ALL market size in the 7MM is expected to change during the study period 2017–2030. The therapeutic market of Acute Lymphoblastic Leukemia in the seven major markets was USD 1,246 million in 2017 which is expected to increase during study period (2017–2030). According to the estimates, the highest market size of Acute Lymphoblastic Leukemia is found in the United States followed by Germany and France.
The United States Market Outlook
In 2017, the total market size of Acute Lymphoblastic Leukemia therapies was USD 907 million in the United States which is expected to increase in the study period (2017–2030).
EU-5 Countries: Market Outlook
In 2017, the total market size of Acute Lymphoblastic Leukemia therapies was USD 282 million in the EU-5 countries which is expected to increase in the study period (2017–2030).
Japan Market Outlook
The total market size of Acute Lymphoblastic Leukemia therapies in Japan was USD 57 million in 2017 which is expected to increase in the study period (2017–2030).
Acute Lymphoblastic Leukemia Drugs Uptake
CAR T cell therapies as a class expected to take major patient share by 2030 in third line and above setting. Around 5 CAR T cell therapies are expected to enter market by 2023 and set to impact the market of current Acute Lymphoblastic Leukemia treatment options mainly stem cell transplantation and Kymriah in third line and above setting. Also, intense competition is anticipated among them which might impact the uptake of these therapies.
Acute Lymphoblastic Leukemia Pipeline Development Activities
The report provides insights into different therapeutic candidates in Phase II, and Phase III stage. It also analyses Acute Lymphoblastic Leukemia key players involved in developing targeted therapeutics.
Acute Lymphoblastic Leukemia Pipeline Development Activities
The Acute Lymphoblastic Leukemia drugs which are in pipeline include:
- KTE-X19 (Gilead Sciences)
- UCART19 (Servier/Allogene)
- Lisocabtagene Maraleucel/JCAR017 (Bristol-Myers Squibb)
- Venetoclax/Venclexta/ABT199/RG7601 (AbbVie and Roche)
- JZP-458/PF743/recombinant Erwinia asparaginase (Jazz Pharmaceuticals)
- Daratumumab (Janssen Research & Development)
- Imbruvica/Ibrutinib {Pharmacyclics (an AbbVie Company)}
- AUTO1 (Autolus Limited)
- PBCAR0191 (Precision BioSciences/Servier)
Access and Reimbursement Scenario in Acute Lymphoblastic Leukemia Therapies
- Acute Lymphoblastic Leukemia is a type of cancer that develops in white blood cells. Over the last few years, the treatment paradigm of Acute Lymphoblastic Leukemia is changed due to the launch of a number of therapies that have improved treatment outcomes for patients with Acute Lymphoblastic Leukemia such as Blincyto and Besponsa; and the first chimeric antigen receptor (CAR) T-cells for relapsed/refractory pediatric and young adult Acute Lymphoblastic Leukemia patients, namely Kymriah. However, chemotherapies are highly effective and remain the backbone of frontline Acute Lymphoblastic Leukemia treatment.
- The market access and reimbursement of advanced therapy medicinal products (ATMPs) (e.g., Kymriah) is difficult due to its high cost, but these therapies offer ground-breaking new opportunities for the treatment of disease. Two thousand nineteen (2019) was a milestone year for CAR-T cell therapy as the product’ manufacturer Novartis (Kymriah) managed to successfully obtain reimbursement in many key countries. The launch of kymriah was highly anticipated by patients, medical professionals, healthcare system stakeholders such as Health Technology Assessment (HTA) bodies and payers, as well as by the wider pharmaceutical industry.
- In France, Kymriah was made available to French patients prior to their European MA through the early access program ‘Temporary Authorisation for Use’ (Autorisation Temporaire d’Utilisation, [ATU]). The ATU route provides reimbursed access before MA approval to therapies that hold particular therapeutic promise and are not currently available through clinical trials in France. After MA, the drug is reimbursed as ‘post-ATU’ until reimbursement and pricing decisions are finalized. During the ATU/post-ATU period, the manufacturers set the drug price freely, however, the pricing committee sets a maximum price per unit. In addition, drugs with annual pre-tax revenue exceeding Euro 30 million under the ATU/post-ATU period are subject to spending caps, above which manufacturers are liable to pay rebates.
KOL- Views
To keep up with current market trends, we take KOLs and SME's opinion working in Acute Lymphoblastic Leukemia (ALL) domain through primary research to fill the data gaps and validate our secondary research. Their opinion helps to understand and validate current and emerging therapies treatment patterns or Acute Lymphoblastic Leukemia market trend. This will support the clients in potential upcoming novel treatment by identifying the overall scenario of the market and the unmet needs.
Competitive Intelligence Analysis
We perform Competitive and Market Intelligence analysis of the Acute Lymphoblastic Leukemia Market by using various Competitive Intelligence tools that include - SWOT analysis, PESTLE analysis, Porter's five forces, BCG Matrix, Market entry strategies etc. The inclusion of the analysis entirely depends upon the data availability.
Scope of the Report
- The report covers the descriptive overview of Acute Lymphoblastic Leukemia, explaining its causes, signs and symptoms, pathophysiology, diagnosis and currently available therapies
- Comprehensive insight has been provided into the Acute Lymphoblastic Leukemia epidemiology and Acute Lymphoblastic Leukemia treatment in the 7MM.
- Additionally, an all-inclusive account of both the current and emerging therapies for Acute Lymphoblastic Leukemia are provided, along with the assessment of new therapies, which will have an impact on the Acute Lymphoblastic Leukemia current treatment landscape
- A detailed review of Acute Lymphoblastic Leukemia market; historical and forecasted is included in the report, covering drug outreach in the 7MM
- The report provides an edge while developing business strategies, by understanding trends shaping and driving the global Acute Lymphoblastic Leukemia market
Report Highlights
- In the coming years, Acute Lymphoblastic Leukemia market is set to change due to the rising awareness of the disease, and incremental healthcare spending across the world; which would expand the size of the market to enable the drug manufacturers to penetrate more into the market
- The companies and academics are working to assess challenges and seek opportunities that could influence Acute Lymphoblastic Leukemia R&D. The therapies under development are focused on novel approaches to treat/improve the disease condition
- Major players are involved in developing therapies for Acute Lymphoblastic Leukemia (ALL). Launch of emerging therapies will significantly impact the Acute Lymphoblastic Leukemia market
- A better understanding of disease pathogenesis will also contribute to the development of novel therapeutics for Acute Lymphoblastic Leukemia
- Our in-depth analysis of the pipeline assets across different stages of development (Phase III and Phase II), different emerging trends and comparative analysis of pipeline products with detailed clinical profiles, key cross-competition, launch date along with product development activities will support the clients in the decision-making process regarding their therapeutic portfolio by identifying the overall scenario of the research and development activities
Acute Lymphoblastic Leukemia (ALL) Report Insights
- Patient Population
- Therapeutic Approaches
- Acute Lymphoblastic Leukemia Pipeline Analysis
- Acute Lymphoblastic Leukemia Market Size and Trends
- Acute Lymphoblastic Leukemia Market Opportunities
- Impact of Acute Lymphoblastic Leukemia upcoming Therapies
Acute Lymphoblastic Leukemia (ALL) Report Key Strengths
- 11 Years Forecast
- Acute Lymphoblastic Leukemia 7MM Coverage
- Acute Lymphoblastic Leukemia Epidemiology Segmentation
- Key Cross Competition
- Highly Analyzed Acute Lymphoblastic Leukemia Market
- Acute Lymphoblastic Leukemia Drugs Uptake
Acute Lymphoblastic Leukemia (ALL) Report Assessment
- Current Treatment Practices
- Unmet Needs
- Acute Lymphoblastic Leukemia Pipeline Product Profiles
- Acute Lymphoblastic Leukemia Market Attractiveness
- Acute Lymphoblastic Leukemia Market Drivers and Acute Lymphoblastic Leukemia Market Barriers
Key Questions
Market Insights:
- What was the Acute Lymphoblastic Leukemia Market share (%) distribution in 2017 and how it would look like in 2030?
- What would be the Acute Lymphoblastic Leukemia total market size as well as market size by therapies across the 7MM during the study period (2017–2030)?
- What are the key findings pertaining to the market across the 7MM and which country will have the largest Acute Lymphoblastic Leukemia market size during the study period (2017–2030)?
- At what CAGR, the Acute Lymphoblastic Leukemia market is expected to grow in the 7MM during the study period (2017–2030)?
- What would be the Acute Lymphoblastic Leukemia market outlook across the 7MM during the study period (2017–2030)?
- What would be the Acute Lymphoblastic Leukemia market growth till 2030 and what will be the resultant market size in the year 2030?
- How would the Acute Lymphoblastic Leukemia market drivers, barriers and future opportunities affect the market dynamics and a subsequent analysis of the associated trends?
- Acute Lymphoblastic Leukemia patient types/pool where unmet need is more and whether emerging therapies will be able to address the residual unmet need?
- How emerging therapies are performing on the parameters like efficacy, safety, route of administration (RoA), Acute Lymphoblastic Leukemia treatment duration and frequencies on the basis of their clinical trial results?
- Among the emerging therapies, what are the potential therapies which are expected to disrupt the Acute Lymphoblastic Leukemia market?
Epidemiology Insights:
- What is the disease risk, burden and unmet needs of the Acute Lymphoblastic Leukemia (ALL)?
- What is the historical Acute Lymphoblastic Leukemia patient pool in seven major markets covering the United States, EU5 (Germany, Spain, France, Italy, UK), and Japan?
- What would be the forecasted patient pool of Acute Lymphoblastic Leukemia in seven major markets covering the United States, EU5 (Germany, Spain, France, Italy, UK), and Japan?
- What will be the growth opportunities in the 7MM with respect to the Acute Lymphoblastic Leukemia patient population?
- Out of all 7MM countries, which country would have the highest prevalent population of Acute Lymphoblastic Leukemia during the forecast period (2017-2030)?
- At what CAGR the population is expected to grow in 7MM during the forecast period (2017-2030)?
Current Treatment Scenario, Marketed Drugs and Emerging Therapies:
- What are the current options for the Acute Lymphoblastic Leukemia treatment, along with the approved therapy?
- What are the current treatment guidelines for the treatment of Acute Lymphoblastic Leukemia in the USA, Europe, and Japan?
- What are the Acute Lymphoblastic Leukemia marketed drugs and their MOA, regulatory milestones, product development activities, advantages, disadvantages, safety and efficacy, etc.?
- How many companies are developing therapies for the treatment of Acute Lymphoblastic Leukemia?
- How many therapies are developed by each company for Acute Lymphoblastic Leukemia treatment?
- How many are emerging therapies in mid-stage, and late stage of development for Acute Lymphoblastic Leukemia treatment?
- What are the key collaborations (Industry - Industry, Industry - Academia), Mergers and acquisitions, licensing activities related to the Acute Lymphoblastic Leukemia therapies?
- What are the recent novel therapies, targets, mechanisms of action and technologies developed to overcome the limitation of existing therapies?
- What are the clinical studies going on for Acute Lymphoblastic Leukemia (ALL) and their status?
- What are the key designations that have been granted for the emerging therapies for Acute Lymphoblastic Leukemia (ALL)?
- What are the global historical and forecasted market of Acute Lymphoblastic Leukemia (ALL)?
Reasons to buy
- The report will help in developing business strategies by understanding trends shaping and driving the Acute Lymphoblastic Leukemia market
- To understand the future market competition in the Acute Lymphoblastic Leukemia market and Insightful review of the key market drivers and barriers
- Organize sales and marketing efforts by identifying the best opportunities for Acute Lymphoblastic Leukemia in the US, Europe (Germany, Spain, Italy, France, and the United Kingdom) and Japan
- Identification of strong upcoming players in the market will help in devising strategies that will help in getting ahead of competitors
- Organize sales and marketing efforts by identifying the best opportunities for Acute Lymphoblastic Leukemia market
- To understand the future market competition in the Acute Lymphoblastic Leukemia market


-market.png&w=256&q=75)
-pipeline.png&w=256&q=75)

