Age-Related Macular Degeneration Market
- The Age-related Macular Degeneration market size in seven major markets is segregated into two part, Dry-AMD market, and Wet-AMD market. As per our analysis and estimation, in 2021, the Age-related Macular Degeneration market size was approximately USD 9,840 million which is further expected to increase by 2034.
- As per our estimation, in 2021, the Age-related Macular Degeneration Market Size was approximately USD 1,377 million, and of Wet AMD was approximately USD 8,463 million which is further expected to increase by 2034.
- The expected launch of potential therapy may increase Age-related Macular Degeneration market size in the coming years, assisted by an increase in the diagnosed prevalent population of Age-related Macular Degeneration.
- Upcoming Age-related Macular Degeneration therapies such as OPT-302, KSI-301, RGX-314, AKST4290, and others has the potential to create a significant positive shift in the Wet-AMD market size.
Request a sample to unlock the CAGR for "Age-related Macular Degeneration Drug Market"
DelveInsight’s ‘Age-related Macular Degeneration (AMD) Market Insights, Epidemiology, and Market Forecast–2034’ report deliver an in-depth understanding of the Age-related Macular Degeneration, historical and forecasted epidemiology as well as the Age-related Macular Degeneration market trends in the United States, EU5 (Germany, France, Italy, Spain, and the United Kingdom) and Japan.
The age-related macular degeneration market report provides current treatment practices, emerging drugs, market share of the individual therapies, current and forecasted 7MM age-related macular degeneration market size from 2020 to 2034.
The AMD Market Report also covers current age-related macular degeneration treatment practice, market drivers, market barriers, SWOT analysis, reimbursement, market access, and unmet medical needs to curate the best of the opportunities and assesses the underlying potential of the AMD treatment market.
|
Study Period |
2020 to 2034 |
|
Forecast Period |
2024-2034 |
|
Geographies Covered |
The US, EU4 (Germany, France, Italy, and Spain) and UK, Japan |
|
Age-related Macular Degeneration Market |
|
|
Age-related Macular Degenerations Market Size | |
|
Age-related Macular Degeneration Companies |
Unity Biotechnology, Inc, PanOptica, Inc., Clearside Biomedical, Alexion Pharmaceuticals, AstraZeneca, Regeneron Pharmaceuticals, Novartis, Roche, Opthea Limited, Kodiak Sciences Inc., REGENXBIO, Alkahest Inc, Graybug Vision, Ribomic USA Inc, Outlook Therapeutics, Inc., Evergreen Therapeutics, Alkeus Pharmaceuticals, Stealth BioTherapeutics, CellCure Neurosciences, Regenerative Patch Technologies, Allegro Ophthalmics, Annexon Biosciences, NGM Biopharmaceuticals, Ionis Pharmaceuticals, Apellis Pharmaceuticals, Iveric Bio, Gyroscope Therapeutics, Luxa Biotechnology, Gemini Therapeutics, and others. |
|
Age-related Macular Degeneration Epidemiology Segmentation |
|
AMD Treatment Market
Age-related Macular Degeneration (AMD) is the leading cause of blindness in elderly patients in developed communities. It is an eye disease of elderly individuals that causes a progressive loss of the central vision needed to drive, read, recognize faces, and see the world in color. AMD's progression leads to loss of central vision, leaving many patients unable to read, write, or recognize both color and detail, thus compromising the quality of life. Although AMD's exact functional pathogenesis is not fully understood, there have been recent improvements in genetic technologies leading to the identification of various polymorphisms that have shown to harbor unique associations with AMD.
Age-related Macular Degeneration is characterized by progressive degeneration of the macula, the central part of the retina, leading to central vision loss. Age-related Macular Degeneration can be classified as early, intermediate, or late based on its clinical features, including drusen, pigmentation abnormalities, atrophy of the retinal pigment epithelium (RPE), and exudative choroidal neovascularization (CNV).
Age-related Macular Degeneration can also be characterized as either dry (atrophic or non-neovascular) or wet (exudative or neovascular).
Age-related Macular Degeneration Diagnosis
AMD rarely causes symptoms in its early stages, annual eye examinations are key to detecting the disease and starting treatments when they are most effective. During an eye exam, the eye healthcare provider checks for changes to the retina and macula. Patients may get one or more of these tests:
Request a sample to unlock the CAGR for Age-related Macular Degeneration Market.
- Visual field test: An Amsler grid has a grid of straight lines with a large dot in the center. The healthcare provider asks to identify lines or sections on the grid that look blurry, wavy, or broken. A lot of distortion may indicate that patient have AMD or the disease is worsening. Patient can use this visual field test at home to monitor vision.
- Dilated eye exam: Eye drops dilate, or widen, patient pupils. Once patient’s eyes are dilated, healthcare provider uses a special lens to look inside patient’s eyes.
- Fluorescein angiography (FA): Healthcare provider injects a yellow dye called fluorescein into a vein in patient’s arm. A special camera tracks the dye as it travels through blood vessels in the eye. The photos can reveal any leakage under the macula.
- Optical coherence tomography (OCT): This imaging machine takes detailed images of the back of the eye, including the retina and macula. Optical coherence tomography isn’t invasive or painful. Patient simply look into a lens while the machine takes pictures.
- Optical coherence tomography angiography (OCTA): This diagnostic tool uses laser light reflection (instead of fluorescein dye) and the OCT scanning device. It takes just a few moments and produces 3D images of blood flow through the eye.
Also, Read @ OPT-302 Drug Insight
Age-related Macular Degeneration Treatment
People rarely lose all of their vision from age-related macular degeneration. Their central vision might be bad, but they’re still able to do many normal daily activities. Usually, patient are also able to use peripheral vision. The dry age-related macular degeneration (dAMD) tends to get worse slowly. There is no cure for macular degeneration. Treatment may slow it down or keep the loss of vision. A large number of studies found have shown, that people with dry AMD could slow the disease by taking supplements of vitamins C and E, lutein, zeaxanthin, zinc, and copper.
Wet age-related macular degeneration (wAMD) can be treated with injections of angiogenesis inhibitors into the eye, photodynamic therapy, or laser surgery. None of these treatments will cure wAMD, but each may slow the vision decline rate or stop further vision loss. However, the disease and loss of vision may also progress despite treatment.
Continued in the report…..
Age-related Macular Degeneration Epidemiology
The disease epidemiology covered in the AMD Market report provides historical as well as forecasted epidemiology segmented by total Age-related Macular Degeneration prevalent cases, diagnosed prevalent cases of AMD, Age-related Macular Degeneration type-specific cases (Dry AMD, and Wet AMD), total age-specific cases of AMD (Dry AMD and Wet AMD), total diagnosed dry AMD cases by stages, and total geographic atrophy cases by visual impairment in the scenario of Age-related Macular Degeneration in the 7MM covering the United States, EU5 countries (Germany, France, Italy, Spain, and the United Kingdom) and Japan from 2020 to 2034.
Key Findings
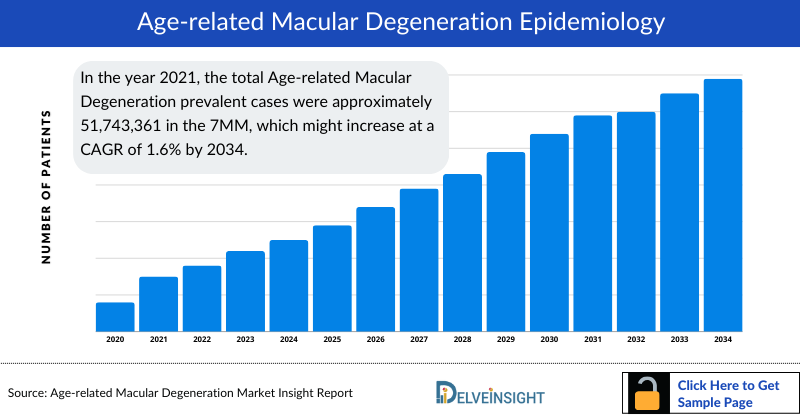
- In the year 2021, the total Age-related Macular Degeneration Prevalence was approximately 51,743,361 in the 7MM, which might increase at a CAGR of 1.6% by 2034.
- The US accounted for approximately 17,282,569 Age-related Macular Degeneration prevalent cases in the year 2021.
- In EU-5 countries the highest number of Age-related Macular Degeneration prevalent cases was observed in Germany with 7,850,793 cases in the year 2021, followed by France.
- Among the 7MM, Japan accounted for approximately 21% prevalent cases of AMD in the year 2021.
- In the year 2021, the total diagnosed cases of AMD were approximately 38,807,521 cases in the 7MM. The highest number of cases were diagnosed in the US.
- In the 7MM, the analysis of type-specific cases of AMD revealed that there were approximately 3,880,752 and 34,926,768 cases of Wet-AMD and Dry-AMD, respectively in the year 2021. These cases are expected to increase by multiple number of folds by 2034.
- In the epidemiology model, the total age-specific cases of AMD (Dry-AMD, and Wet-AMD), are divided into age group 50-59 years, 60–69 years, 70–79 years, and ≥80 years respectively. As per the estimates by DelveInsight, the total age-specific cases of AMD are expected to increase during the forecasted period of 2022–2034.
- In the US, the total diagnosed cases of dry AMD by age distribution were 2,175,435; 3,380,209; 3,495,783; and 2,614,307 cases for the age group 50–59 years, 60–69 years, 70–79 years, and ≥ 80 years in 2021.
- In the US, the total diagnosed cases of dry AMD by stages were approximately 10,499,160 cases for early and intermediate dry AMD and 1,166,573 cases for advanced (geographic atrophy associated with dry AMD) in 2021. As per the analysis, stage-wise cases are also expected to by 2034.
- In the US, the total geographic atrophy cases by visual impairment were 349,972; 583,287; and 233,315 cases for mild, moderate to severe, and patients going for surgery or having legal blindness, respectively, in 2021.
Country-Wise Age-related Macular Degeneration Epidemiology
The epidemiology segment also provides the Age-related Macular Degeneration epidemiology data and findings across the United States, EU5 (Germany, France, Italy, Spain, and the United Kingdom), and Japan.
Read the full report @ Age-related Macular Degeneration Prevalence
Recent Developments in Age-related Macular Degeneration Clinical Trials
- In February 2025, Astellas Pharma announced that the FDA approved expanded prescribing information for IZERVAY™ (avacincaptad pegol intravitreal solution) for treating geographic atrophy (GA) secondary to age-related macular degeneration (AMD). The approval removes the limitation on the duration of dosing, offering physicians and patients more flexibility in managing geographic atrophy.
- In February 2025, Astellas Pharma Inc. announced that the FDA approved expanded U.S. prescribing information for IZERVAY™ (avacincaptad pegol intravitreal solution) for the treatment of geographic atrophy (GA) secondary to age-related macular degeneration (AMD).
- In Jan 2025, Astellas Pharma announced that the U.S. FDA accepted the Class 1 resubmission of the supplemental New Drug Application for IZERVAY, a treatment for geographic atrophy associated with age-related macular degeneration.
Age-related Macular Degeneration Drug Chapters
The drug chapter segment of the Age-related Macular Degeneration drugs market report encloses the detailed analysis of Age-related Macular Degeneration marketed drugs, mid-phase, and late-stage pipeline drugs. It also helps to understand the Age-related Macular Degeneration clinical trial details, expressive pharmacological action, agreements and collaborations, approval, and patent details of each included drug, and the latest news and press releases.
Marketed Age-related Macular Degeneration Drugs
- Eylea (aflibercept): Regeneron Pharmaceuticals
Eylea developed by Regeneron is used to treat wet-AMD. It is also used to treat diabetic eye disease and other problems of the retina. It is injected into the eye to help slow vision loss from these diseases. Eylea is the brand name for the drug called aflibercept. VEGF, a naturally occurring protein in the body triggers angiogenesis, however, in wet-AMD it is associated with abnormal blood vessel growth which causes edema leading to vision loss. Eylea blocks the growth of abnormal blood vessels in the back of the eye.
At present Regeneron is conducting phase III trials to explore less frequent dosing intervals of Eylea. It is evaluating the drug for a higher dose of 8mg in wet-AMD patients after phase II trials met its primary endpoint.
Products detail in the report…
- Beovu (brolucizumab): Novartis
Beovu (brolucizumab) is the most clinically advanced humanized single-chain antibody fragment (scFv). Single-chain antibody fragments are highly sought after in drug development due to their small size, enhanced tissue penetration, rapid clearance from systemic circulation, and drug delivery characteristics.
Beovu is the first FDA-approved anti-VEGF to offer both greater fluid resolution versus aflibercept and the ability to maintain eligible wet AMD patients on a three-month dosing interval immediately after a three-month loading phase with uncompromised efficacy.
Products detail in the report…
- Lucentis (ranibizumab): Roche
Lucentis (ranibizumab) is a drug used to treat wet age-related macular degeneration (AMD). It is also used to treat diabetic eye disease and other problems of the retina. It is injected into the eye to help slow vision loss from these diseases. Lucentis is the brand name for the drug, which is called ranibizumab. Ranibizumab is a humanized recombinant monoclonal antibody fragment targeted against human vascular endothelial growth factor A (VEGF-A). It binds with high affinity to the VEGF-A isoforms (e.g. VEGF110, VEGF121, and VEGF165), thereby preventing the binding of VEGF-A to its receptors VEGFR-1 and VEGFR-2
Know More @ KSI-301 Drug Insight
Emerging Age-related Macular Degeneration Drugs
- OPT-302: Opthea Limited
OPT-302 (sVEGFR-3) is the first ‘Trap’ inhibitor of VEGF-C and VEGF-D designed specifically for the eye. OPT-302 blocks the two members of the VEGF family which cause blood vessels to grow and leak. Aberrant blood vessel growth and vascular leakage are hallmarks of several eye diseases, including wet AMD and Diabetic Macular Edema. In combination with anti-VEGF-A therapies, OPT-302 completely shuts-down VEGFR-2 and VEGFR-3 activity and targets mechanisms of resistance and suboptimal clinical response to existing therapies. The company is currently conducting two concurrent Phase III clinical trials of OPT-302 for the treatment of wet AMD.
Products detail in the report…
- KSI-301: Kodiak Sciences Inc.
KSI-301 is a novel anti-VEGF biologic designed to rapidly inhibit VEGF and provide extended durability of action to reduce the burden of frequent anti-VEGF injections. Delivering potent and sustained VEGF inhibition enables patient compliance, results in long-term efficacy, and improves visual acuity outcomes.The drug is currently being evaluated in Phase III. The readouts of the trial are expected in quarter one of 2023.
Products detail in the report…
- RGX-314: Regenxbio
RGX-314 is being developed as a novel, one-time subretinal treatment that includes the NAV AAV8 vector containing a gene encoding for a monoclonal antibody fragment. The expressed protein is designed to neutralize vascular endothelial growth factor (VEGF) activity, modifying the pathway for the formation of new leaky blood vessels and retinal fluid accumulation. It is a potential one-time gene therapy for the treatment of wet AMD, diabetic retinopathy (DR) and other chronic retinal diseases.RGX-314 is currently being evaluated in patients with wAMD in Phase II and II/III clinical trials.
Products detail in the report…
- Danicopan (ALXN2040): Alexion Pharmaceuticals and AstraZeneca
Danicopan (ALXN2040) is an oral drug developed by Alexion. It is an investigational first-in-class small-molecule inhibitor of factor D, an essential enzyme of the complement alternative pathway, which, when dysregulated, is implicated in the pathogenesis of AMD.The company is conducting a Phase II study in patients with Geographic Atrophy, secondary to AMD, and data is expected in 2023 plus.
Products detail in the report…
- EG-301: Evergreen Therapeutics
EG-301 is an oral dosage form that is effective and safe in animal studies with fewer side effects and is used to treat patients over 50 years of age with dry macular disease. The company is currently conducting a Phase II study to treat non-exudative (dry) AMD.
Products detail in the report…
List of products to be continued in the report…
Age-related Macular Degeneration Market Outlook
Age-related Macular Degeneration (AMD) is a chronic, progressive, and severe disease of the central retina and is the main cause of irreversible blindness in the Western world and Asia-Pacific countries. Up to date, 8.7% of the worldwide population has AMD. The wet form of the disease is responsible for more than 90% of the severe central visual acuity (VA) loss associated with AMD.
Wet AMD Market Outlook
The introduction of anti-VEGF intravitreal injections has opened a new therapeutic window in the management of wet AMD, thus efficiently blocking the pathophysiological process of AMD, with a restoration of retinal morphology and the maintenance of its function. In the past years, anti-VEGF injections have become the standard treatment for wet AMD, accounting for better results than the previous choices, such as photodynamic therapy (PDT) and laser photocoagulation. Currently, drugs like Lucentis (ranibizumab), Eylea (aflibercept), and Avastin (bevacizumab), work well to achieve a rapid resolution of exudative signs in most patients. Faricimab and Susvimo have also been recently approved for the treatment of Wet AMD.
Dry AMD Market Outlook
At present, the therapeutic landscape of dry AMD is devoid of any approved treatment, and to manage this indication, and there is a substantial unmet need for a therapy to slow its worsening. The pathophysiology of dry AMD is poorly understood. However, there are chances of failure to reach the primary outcomes that might be linked to the advanced stage of the disease rather than a lack of action on appropriate targets. The forecast model does not include other therapy options since DelveInsight’s analysis does not consider procedures and lifestyle management therapy options for predicting the market size in the 7MM countries. The market size solely focuses on the revenue generated by the pharmacological treatment of metal poisoning.
You may read this report @ RGX-314 Drug Insight
Key Findings
- The Age-related Macular Degeneration market size in seven major markets is segregated into two part, Dry-AMD, and Wet-AMD. As per our analysis and estimation, in 2021, the Age-related Macular Degeneration market size was approximately USD 9,840 million which is further expected to increase by 2034.
- As per our estimation, in 2021, the Dry AMD market size was approximately USD 1,377 million, and of Wet AMD market size was approximately USD 8,463 million which are further expected to increase by 2034.
- The expected launch of potential therapy may increase the Age-related Macular Degeneration market size in the coming years, assisted by an increase in the diagnosed prevalent population of Age-related Macular Degeneration.
- Upcoming AMD therapy such as OPT-302, KSI-301, RGX-314, AKST4290, and others has the potential to create a significant positive shift in the Wet-AMD market size.
- A significant positive shift in the Dry-AMD market size is also expected due to the increased prevalence and launch of upcoming Dry-AMD therapies during the forecast period.
- The United States accounts for the largest Age-related Macular Degeneration market size, in comparison to EU5 (Germany, Italy, France, Spain, and the United Kingdom) and Japan.
The United States: Age-related Macular Degeneration Market Outlook
This section provides the total Age-related Macular Degeneration market size and market size by therapies in the United States.
EU-5: Age-related Macular Degeneration Market Outlook
The total Age-related Macular Degeneration market size and market size by therapies in Germany, France, Italy, Spain, and the United Kingdom are provided in this section.
Japan: Age-related Macular Degeneration Market Outlook
The total Age-related Macular Degeneration market size and market size by therapies in Japan are provided.
Age-related Macular Degeneration Drugs Uptake
This section focuses on the rate of uptake of the potential Age-related Macular Degeneration drugs recently launched in the Age-related Macular Degeneration market or expected to get launched in the market during the study period 2020–2034. The analysis covers the Age-related Macular Degeneration market uptake by drugs; patient uptake by therapies; and sales of each drug.
This helps in understanding the drugs with the most rapid uptake, and reasons behind the maximal use of new Age-related Macular Degeneration drugs and allows, the comparison of the drugs based on Age-related Macular Degeneration market share and size which again will be useful in investigating factors important in AMD market uptake and in making financial and regulatory decisions.
Age-related Macular Degeneration Development Activities
The Age-related Macular Degeneration pipeline segment provides insights into different therapeutic candidates in phase II, and phase III stages also analyze Age-related Macular Degeneration companies involved in developing targeted therapeutics.
Development Activities
The Age-related Macular Degeneration pipeline segment covers the detailed information of collaborations, acquisition, and merger, licensing, and patent details for Age-related Macular Degeneration therapies.
Read the full report @ New Age-related Macular Degeneration Drugs
Reimbursement Scenario
Approaching reimbursement proactively can have a positive impact both during the late stages of product development and well after product launch. In the report, we consider reimbursement to identify economically attractive indications and market opportunities. When working with finite resources, the ability to select the markets with the fewest reimbursement barriers can be a critical business and price strategy.
Competitive Intelligence Analysis
We perform competitively and market Intelligence analysis of the Age-related Macular Degeneration market by using various competitive intelligence tools that include–SWOT analysis, PESTLE analysis, Porter’s five forces, BCG Matrix, Market entry strategies, etc. The inclusion of the analysis entirely depends upon the data availability.
Age-related Macular Degeneration Market Research Report Scope
- The Age-related Macular Degeneration market report covers the descriptive overview of Age-related Macular Degeneration, explaining its etiology, signs and symptoms, pathophysiology, genetic basis, and currently available therapies.
- Comprehensive insight has been provided into the Age-related Macular Degeneration epidemiology and treatment.
- Additionally, an all-inclusive account of both the current and emerging therapies for Age-related Macular Degeneration is provided, along with the assessment of new therapies, which will have an impact on the current treatment landscape.
- A detailed review of the Age-related Macular Degeneration market; historical and forecasted is included in the report, covering the 7MM drug outreach.
- The patient-based Age-related Macular Degeneration market forecasting report provides an edge while developing business strategies, by understanding trends shaping and driving the 7MM Age-related Macular Degeneration market.
Age-related Macular Degeneration Market Research Report Highlights
- The robust pipeline with novel MOA and oral ROA and increasing diagnosed prevalence will positively drive the Age-related Macular Degeneration market.
- The Age-related Macular Degeneration companies and academics are working to assess challenges and seek opportunities that could influence Age-related Macular Degeneration R&D. The therapies under development are focused on novel approaches to treat/improve the disease condition.
- Major AMD Companies are involved in developing therapies for Age-related Macular Degeneration. The launch of emerging therapies will significantly impact the Age-related Macular Degeneration market.
- Our in-depth analysis of the pipeline assets across different stages of development (phase III and phase II), different emerging trends, and comparative analysis of pipeline products with detailed clinical profiles, key cross-competition, launch date along with product development activities will support the clients in the decision-making process regarding their therapeutic portfolio by identifying the overall scenario of the research and development activities.
Age-related Macular Degeneration Market Forecast Report Insights
- Patient-based Age-related Macular Degeneration Market Forecasting
- Age-related Macular Degeneration Therapeutic Approaches
- Age-related Macular Degeneration Pipeline Analysis
- Age-related Macular Degeneration Market Size
- Age-related Macular Degeneration Market Trends
- Age-related Macular Degeneration Market Opportunities
- Impact of upcoming Age-related Macular Degeneration Therapies
Age-related Macular Degeneration Treatment Market Report Key Strengths
- 10 Years- Age-related Macular Degeneration Market Forecast
- The 7MM Coverage
- Age-related Macular Degeneration Epidemiology Segmentation
- Key Cross Competition
- Highly Analyzed Age-related Macular Degeneration Market
- Age-related Macular Degeneration Drugs Uptake
Age-related Macular Degeneration Treatment Market Report Assessment
- Current Age-related Macular Degeneration Treatment Market Practices
- Age-related Macular Degeneration Unmet Needs
- Age-related Macular Degeneration Pipeline Drugs Profiles
- Age-related Macular Degeneration Market Attractiveness
- Age-related Macular Degeneration Market Drivers and Barriers
- SWOT analysis
Key Questions
Age-related Macular Degeneration Treatment Market Insights:
- What was the Age-related Macular Degeneration market share (%) distribution in 2020 and how it would look like in 2034?
- What would be the Age-related Macular Degeneration market size as well as market size by therapies across the 7MM during the forecast period (2023–2034)?
- What are the key findings pertaining to the market across the 7MM and which country will have the largest Age-related Macular Degeneration market size during the forecast period (2023–2034)?
- At what CAGR, the Age-related Macular Degeneration market is expected to grow at the 7MM level during the forecast period (2023–2034)?
- What would be the Age-related Macular Degeneration market outlook across the 7MM during the forecast period (2023–2034)?
- What would be the Age-related Macular Degeneration market growth till 2034 and what will be the resultant market size in the year 2034?
- How would the market drivers, barriers, and future opportunities affect the Age-related Macular Degeneration market dynamics and subsequent analysis of the associated trends?
Age-related Macular Degeneration Epidemiology Insights:
- What is the disease risk, burden, and Age-related Macular Degeneration unmet needs?
- What is the historical Age-related Macular Degeneration patient pool in the United States, EU5 (Germany, France, Italy, Spain, and the UK), and Japan?
- What would be the forecasted patient pool of Age-related Macular Degeneration at the 7MM level?
- What will be the growth opportunities across the 7MM with respect to the patient population pertaining to Age-related Macular Degeneration?
- Out of the above-mentioned countries, which country would have the highest population of Age-related Macular Degeneration during the forecast period (2022–2034)?
- At what CAGR the population is expected to grow across the 7MM during the forecast period (2022–2034)?
Current Age-related Macular Degeneration Treatment Market Scenario, Marketed Drugs, and Emerging Therapies:
- What are the current options for the treatment of Age-related Macular Degeneration along with the approved therapy?
- What are the current treatment guidelines for the treatment of Age-related Macular Degeneration in the US and Europe?
- What are the Age-related Macular Degeneration marketed drugs and their MOA, regulatory milestones, product development activities, advantages, disadvantages, safety, and efficacy, etc.?
- How many AMD companies are developing therapies for the treatment of Age-related Macular Degeneration?
- How many emerging therapies are in the mid-stage and late stages of development for the treatment of Age-related Macular Degeneration?
- What are the key collaborations (Industry–Industry, Industry-Academia), mergers and acquisitions, licensing activities related to the Age-related Macular Degeneration therapies?
- What are the recent novel therapies, targets, mechanisms of action, and technologies developed to overcome the limitation of existing therapies?
- What are the clinical studies going on for Age-related Macular Degeneration and their status?
- What are the key designations that have been granted for the emerging therapies for Age-related Macular Degeneration?
- What are the 7MM historical and forecasted Age-related Macular Degeneration market?
Reasons to buy
- The patient-based Age-related Macular Degeneration market forecasting report will help in developing business strategies by understanding trends shaping and driving Age-related Macular Degeneration.
- To understand the future market competition in the Age-related Macular Degeneration market and an Insightful review of the key market drivers and barriers.
- Organize sales and marketing efforts by identifying the best opportunities for Age-related Macular Degeneration in the US, Europe (Germany, Spain, Italy, France, and the United Kingdom), and Japan.
- Identification of strong upcoming Age-related Macular Degeneration companies in the AMD Treatment market will help in devising strategies that will help in getting ahead of competitors.
- Organize sales and marketing efforts by identifying the best opportunities for the Age-related Macular Degeneration market.
- To understand the future market competition in the Age-related Macular Degeneration market.
Discover additional resources including Articles and Infographics:-
- Age-Related Macular Degeneration Whitepaper
- Age-Related Macular Degeneration Market: Infographics
- Age-Related Macular Degeneration Market: Video
Unlock Valuable Content @ Latest DelveInsight Blog