Amyotrophic Lateral Sclerosis Market
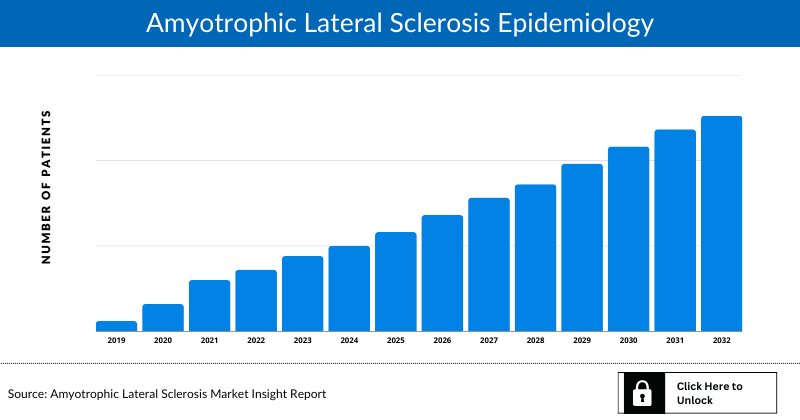
- The total Amyotrophic Lateral Sclerosis Diagnosed Cases was 59,130 cases in the 7MM which are expected to grow during the study period, i.e., 2019–2032
- In 2021, the Amyotrophic Lateral Sclerosis market size was USD 490 million which is expected to rise during the study period (2019–2032).
- The leading companies working in the Amyotrophic Lateral Sclerosis market include Helixmith, Sanofi, Denali Therapeutics, Alector,GSK, NeuroSense Therapeutics, Biogen, Ionis,Brainstorm Cell Therapeutics, Cytokinetics, Astellas Pharma, Apellis Pharmaceuticals, Clene Nanomedicine Biosciences, AL-S Pharma, MediciNova, Seelos Theraputics, Prilenia Therapeutics, AB Science, Eledon Pharmaceuticals, Revalesio Corporation, Biohaven Pharmaceuticals, UCB Pharma, Ra Pharmaceuticals, and others.
Request a sample to unlock the CAGR for Amyotrophic Lateral Sclerosis Market
DelveInsight's "Amyotrophic Lateral Sclerosis Market Insights, Epidemiology, and Market Forecast-2032" report delivers an in-depth understanding of the Amyotrophic Lateral Sclerosis, historical and forecasted epidemiology as well as the Amyotrophic Lateral Sclerosis market trends in the United States, EU-4 (Germany, France, Italy, and Spain), the United Kingdom, Japan and China.
The Amyotrophic Lateral Sclerosis market research report provides current treatment practices, emerging drugs, Amyotrophic Lateral Sclerosis market share of the individual therapies, current and forecasted Amyotrophic Lateral Sclerosis market Size from 2019 to 2032 segmented by seven major markets. The report also covers the current Amyotrophic Lateral Sclerosis treatment market, Amyotrophic Lateral Sclerosis market drivers market barriers and unmet medical needs to curate best of the opportunities and assess the underlying potential of the market.
|
Study Period |
2019 to 2032 |
|
Forecast Period |
2023-2032 |
|
Geographies Covered |
|
|
Amyotrophic Lateral Sclerosis Disorder Market |
|
|
Amyotrophic Lateral Sclerosis Market Size | |
|
Amyotrophic Lateral Sclerosis Companies |
|
Amyotrophic Lateral Sclerosis Treatment Market
Amyotrophic Lateral Sclerosis (ALS), also known as Lou Gehrig’s disease, is a group of rare neurological diseases that mainly involve the nerve cells (neurons) responsible for controlling voluntary muscle movement. The disease is progressive, meaning the symptoms get worse over time. ALS belongs to a wider group of disorders known as motor neuron diseases caused by gradual deterioration (degeneration) and death of motor neurons. Motor neurons are nerve cells that extend from the brain to the spinal cord and muscles throughout the body. Messages from motor neurons in the brain (called upper motor neurons; UMN) are transmitted to motor neurons in the spinal cord and to motor nuclei of the brain (called lower motor neurons; LMN) and from the spinal cord and motor nuclei of the brain to a particular muscle or muscles. ALS can be either sporadic or genetic. The sporadic type is the most common and can affect anyone. The genetic or familial type is rarer. Common symptoms include painless, progressive muscle weakness. The first thing a person might notice is tripping more often, or dropping things because of the weakness. Slurred speech, difficulty swallowing, and trouble breathing can occur.
Amyotrophic Lateral Sclerosis Diagnosis
Amyotrophic Lateral Sclerosis is a difficult disease to diagnose. There is no one test or procedure to establish the diagnosis of Amyotrophic Lateral Sclerosis ultimately. It is through a clinical examination and a series of diagnostic tests, often ruling out other diseases that mimic Amyotrophic Lateral Sclerosis. The diagnosis of Amyotrophic Lateral Sclerosis relies on medical history, physical examination, electrodiagnostic testing (with needle EMG), and neuroimaging. Biomarkers can play a crucial role in diagnostic, prognostic, or predictive research studies. They could potentially become important for the stratification of patients and monitoring treatment effects in clinical trials. Genetic testing of the five most prevalent genes found to be mutated in Amyotrophic Lateral Sclerosis is routinely offered to patients with a positive family history (C9orf72, SOD1, TDP‐43, FUS, and TBK‐1).
Amyotrophic Lateral Sclerosis Treatment
There is no cure for Amyotrophic Lateral Sclerosis, so treatment aims to alleviate symptoms, prevent unnecessary complications, and slow the rate of disease progression. Medical interventions and technology have vastly improved the quality of life for people with Amyotrophic Lateral Sclerosis by assisting with breathing, nutrition, mobility, and communication. Modern therapeutic strategies comprise of neuroprotective treatment focused on antiglutamatergic, antioxidant, antiapoptotic, and anti-inflammatory molecules. Drugs approved for the treatment of Amyotrophic Lateral Sclerosis are IV Radicava (Mitsubishi Tanabe Pharma), Rilutek/Riluzole) (Sanofi/Covis), Exservan (Aquestive Therapeutics), and Tiglutik/ Teglutik (ITF Pharma). In Amyotrophic Lateral Sclerosis, opioids and nonsteroidal anti-inflammatory drugs (NSAIDs) are widely used to relieve discomfort. A combination of quinidine and dextromethorphan called Nuedexta (Avanir Pharmaceuticals) has been approved by the US Food and Drug Administration to treat pseudobulbar effect, however it can lengthen the QT interval, raising the risk of cardiac arrhythmia.
Amyotrophic Lateral Sclerosis Epidemiology
The Amyotrophic Lateral Sclerosis epidemiology division provides insights about historical and current Amyotrophic Lateral Sclerosis patient pool and forecasted trends for every seven major countries. It helps to recognize the causes of current and forecasted trends by exploring numerous studies and views of key opinion leaders. This part of the DelveInsight’s report also provides the diagnosed patient pool and their trends along with assumptions undertaken.
Key Findings
The disease epidemiology covered in the report provides historical as well as forecasted Amyotrophic Lateral Sclerosis epidemiology [segmented Prevalence of Amyotrophic Lateral Sclerosis, Diagnosed Prevalence of Amyotrophic Lateral Sclerosis, Gender-specific Distribution of Amyotrophic Lateral Sclerosis, Mutation-specific Distribution of Amyotrophic Lateral Sclerosis, Type-specific Distribution of Amyotrophic Lateral Sclerosis, Distribution based on Site of onset of Amyotrophic Lateral Sclerosis, Age-specific Distribution of Amyotrophic Lateral Sclerosis in the 7MM and China covering the United States, EU-4 countries (Germany, France, Italy, and Spain), the United Kingdom, Japan, and China from 2019 to 2032.
Amyotrophic Lateral Sclerosis Epidemiology- Country Wise
- Estimates show that the highest cases of Amyotrophic Lateral Sclerosis in the 7MM were in the United States, followed by EU-4 countries (Germany, France, Italy, and Spain), the United Kingdom and Japan in the year 2021.
- The total diagnosed prevalent cases of Amyotrophic Lateral Sclerosis in the 7MM and China were 59,130 and 29,095 in 2021, growing at a CAGR of 1.3% and 0.6% respectively during the study period (2019–2032).
- Epidemiology assessed for the condition showed that the US, in 2021 accounted for approximately 25,817 diagnosed prevalent cases of Amyotrophic Lateral Sclerosis.
- Among the EU-4 and the United Kingdom countries in 2021, Germany had the highest diagnosed prevalent cases of Amyotrophic Lateral Sclerosis with 5,398 cases, followed by UK (4,561) and Italy (4,478). In contrast, Spain had the lowest cases (3,270) in 2021.
- Japan accounted for 11,183 diagnosed prevalent Amyotrophic Lateral Sclerosis cases in 2021.
- In the United States, in 2021, the proportion of male and female cases were 14,948 and 10,869 respectively.
- As per the analysis, a higher percentage of sporadic Amyotrophic Lateral Sclerosis was observed in the 7MM and China compared to familial Amyotrophic Lateral Sclerosis in 2021.
- Amyotrophic Lateral Sclerosis can be divided into C9ORF72, SOD1, FUS, others mutations (TARDBP, OPTN, ANG, etc.), and non-mutated/unidentified mutation based on the types of mutations causing the condition. In the United States, the number of cases of C9ORF72, SOD1, FUS, others mutations (TARDBP, OPTN, ANG, etc.), and non-mutated/unidentified mutation was 2,478, 749, 361, 2,189 and 20,039 respectively, in 2021.
- Japan accounted for approximately 3,892 cases of 70-79 years of age of Amyotrophic Lateral Sclerosis in 2021.
- In China, in 2021 the proportion of male and female cases were 17,948 and 11,146 respectively.
- In 2021, the patients with the spinal site of onset accounted for 21,035 cases, followed by 5,673 cases with the bulbar site of onset, and 2,386 cases with other uncertain regions in China.
Amyotrophic Lateral Sclerosis Recent Developments
- On November 25, 2024, Ractigen Therapeutics announced that its therapy, RAG-21, for amyotrophic lateral sclerosis (ALS) linked to FUS gene mutations, has been granted orphan drug status by the FDA.
Amyotrophic Lateral Sclerosis Drug Chapters
Drug chapter segment of the Amyotrophic Lateral Sclerosis report encloses the detailed analysis of Amyotrophic Lateral Sclerosis marketed drugs and late stage (Phase-III, Phase-II, and Phase I/II) Amyotrophic Lateral Sclerosis pipeline drugs. It also helps to understand the Amyotrophic Lateral Sclerosis clinical trial details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug and the latest news and press releases.
Amyotrophic Lateral Sclerosis Marketed Drugs
- Radicava (Mitsubishi Tanabe Pharma Corporation)
Radicava (edaravone) is a free radical scavengers and administered as intravenous infusion of 60 mg over 60 minutes. Radicava received fast track and orphan drug designation by FDA and Orphan drug designation by EMA. It is approved in the USA and Japan for the treatment of Amyotrophic Lateral Sclerosis. In May 2019, Radicava, Mitsubishi Tanabe Pharma notified the Committee for Medicinal Products for Human Use (CHMP) that it wishes to withdraw its application for a marketing authorisation for Radicava intended for the treatment of Amyotrophic Lateral Sclerosis. The company is currently conducting a Phase III trial for long-term safety and tolerability of oral edaravone for the treatment of Amyotrophic Lateral Sclerosis. MT-1186 is an oral suspension formulation that contains the same active ingredient as edaravone for IV infusion (Japanese product name: RADICUT injection 30 mg and RADICUT bag for IV infusion 30 mg) for Amyotrophic Lateral Sclerosis treatment.
In May 2022, the company announced that FDA has approved RADICAVA ORS (edaravone), the oral form of edaravone, for the treatment of Amyotrophic Lateral Sclerosis, a neurodegenerative disease that currently has no cure and can progress rapidly. RADICAVA ORS offers the same efficacy as RADICAVA (edaravone), an FDA-approved IV treatment shown in a pivotal trial to help slow the loss of physical function in Amyotrophic Lateral Sclerosis. In March 2022, the company submitted an application to MHLW (Japan) for marketing and manufacturing approval of oral formulation of MT-1186 for treating Amyotrophic Lateral Sclerosis.
- RELYVRIO (AMX0035) Amylyx Pharmaceuticals
RELYVRIO is an investigational neuroprotective therapy being developed to minimize neuronal death and dysfunction. In Amyotrophic Lateral Sclerosis and other neurodegenerative disorders, the drug targets endoplasmic reticulum and mitochondrial-dependent neuronal degeneration pathways and blocks stress to maintain a balance between them. In September 2022, the FDA approved RELYVRIO (sodium phenylbutyrate and taurursodiol) for treating adults with Amyotrophic Lateral Sclerosis. AMX0035 is an investigational drug not approved for use by EMA; however, MAA was validated for review by EMA’S CHMP in February 2022, and the potential decision is anticipated by 1H 2023.
- Exservan (Aquestive Therapeutics)
Exservan (Riluzole oral film) consists of a thin film that is placed on the tongue, utilizes the company’s “PharmFilm” technology. The dissolving oral film can be taken twice daily without water, making it easier for patients who have difficulty swallowing pills or liquids. Riluzole oral film received the US FDA Orphan Drug designation which is used as adjunctive therapy in the treatment of Amyotrophic Lateral Sclerosis. Aquestive Therapeutics announced the exclusive license to Mitsubishi Tanabe Pharma Holdings America (MTHA) for the commercialization Exservan in the United States and Zambon for the development and commercialization of Exservan in the European Union for the treatment of Amyotrophic Lateral Sclerosis.
- TIGLUTIK/ TEGLUTIK (ITF Pharma)
TIGLUTIK (riluzole) oral suspension is indicated for the treatment of Amyotrophic Lateral Sclerosis. TIGLUTIK is the first and only easy-to-swallow thickened riluzole liquid for Amyotrophic Lateral Sclerosisand is administered twice daily via an oral syringe. TEGLUTIK was first launched in the European markets after it was approved by US FDA as Tiglutik for the treatment of Amyotrophic Lateral Sclerosis. In 2022, TEGLUTIK (riluzole), the only oral suspension for the treatment of Amyotrophic Lateral Sclerosis, developed and patented by Italfarmaco, has been approved for the Chinese market by the Drug Administration Law of the People's Republic of China.
- Nuedexta (Avanir Pharmaceuticals)
Nuedexta (dextromethorphan hydrobromide/quinidine sulfate) is the only approved medication that has proven to be effective in lowering down the Pseudobulbar Affect (PBA). Nuedexta acts on sigma-1 and NMDA receptors in the brain, although the mechanism by which Nuedexta exerts therapeutic effects in patients with PBA is unknown. It is approved in the USA and Europe for the treatment of PBA associated with certain neurological conditions such as Amyotrophic Lateral Sclerosis.
Note: Detailed Current therapies assessment will be provided in the full report of Amyotrophic Lateral Sclerosis
Amyotrophic Lateral Sclerosis Emerging Drugs
- Tofersen (Biogen/Ionis Pharmaceuticals)
Tofersen also known as BIIB067, is an antisense drug designed to reduce the production of superoxide dismutase 1 (SOD1), which is the best understood genetic cause of familial Amyotrophic Lateral Sclerosis. The drug is being currently investigated in two Phase III trials for the treatment of Amyotrophic Lateral Sclerosis caused by SOD1 mutation. In October 2021, the company presented the Phase III results from VALOR (Part C), which showed that the trial did not meet the primary endpoint. This failure could likely hinder Biogen’s planned filing for FDA approval in the Amyotrophic Lateral Sclerosis market. In Q2 2022, the company presented 12-month data of tofersen in SOD1-Amyotrophic Lateral Sclerosis from the Phase III VALOR study and it’s OLE at the European Network to Cure Amyotrophic Lateral Sclerosis. In July 2022, the US FDA accepted Biogen’s New Drug Application (NDA), filing of tofersen, and an investigational antisense medicine for the treatment of superoxide dismutase 1 amyotrophic lateral sclerosis (SOD1-ALS).
- ION363 (Ionis Pharmaceuticals)
Ionis Pharmaceuticals’ portfolio for Amyotrophic Lateral Sclerosis also includes ION363, another investigational antisense medicine for Amyotrophic Lateral Sclerosis, designed to reduce the Fused in Sarcoma (FUS) protein production. The drug is owned by Ionis and is in development for patients with a rare genetic form of Amyotrophic Lateral Sclerosis caused by mutations in the FUS gene, which causes motor neuron degeneration through a toxic gain of function mechanism. ION363 can potentially reduce or prevent disease progression in FUS-Amyotrophic Lateral Sclerosis patients, and the data from the ongoing Phase III trial is expected in 2024.
- Masitinib (AB Sciences)
Masitinib is an orally administered tyrosine kinase inhibitor, which has already FDA on the IND application. The drug is under investigation for a Phase III trial in patients with Amyotrophic Lateral Sclerosis but was previously put on hold due to a potential risk of ischemic heart disease with masitinib, voluntary by the company – the trial was resumed after the FDA authorization. In 2022, the company announced that Health Canada has granted authorization to file a New Drug Submission for Masitinib in treating Amyotrophic Lateral Sclerosis under the Notice of Compliance with Conditions (NOC/c) policy. In 2022, the company filed an application for conditional Marketing Authorization to the EMA for Alsitek (Masitinib) to treat ALS.
- AIT-101 (LAM-002A) - AI Therapeutics
AIT-101 ((LAM-002A) is a proprietary, oral dissolving formulation of a potent and highly selective PIKfyve kinase inhibitor. The active drug substance in AIT-101 is an experimental therapy that has been evaluated and demonstrated to be safe in almost 1,000 human subjects, including healthy volunteers and patients. In 2022, Company announced that it has launched a Phase II clinical trial into AIT-101 (apilimod dimesylate) as a potential oral treatment for amyotrophic lateral sclerosis (ALS) associated with C9orf72 mutations.
- Pridopidine (Prilenia Therapeutics)
Pridopidine is an investigational sigma-1 receptor agonist for Amyotrophic Lateral Sclerosis treatment. Pridopidine has an established safety profile and therapeutic potential in several neurodegenerative diseases affecting adults and children. In 2022, the company raised an additional USD 10 million, bolstering the Series B financing round and bringing the total capital raised to USD 144 million, pridopidine for patients with Huntington’s disease (HD) and Amyotrophic Lateral Sclerosis through 2024.The data readouts for Prilenia’s clinical trials are expected in the next 12 months. The company anticipates sharing data from the HEALEY ALS Platform Trial by late 2022 and PROOF-HD (Huntington’s disease) topline results by early 2023.
Note: Detailed emerging therapies assessment will be provided in the final report.
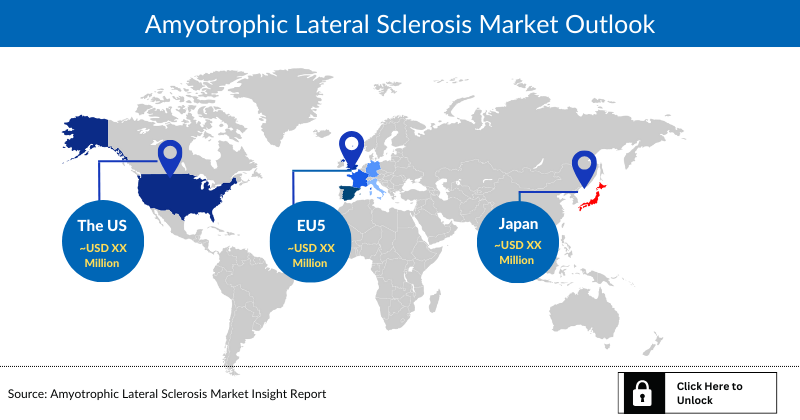
Amyotrophic Lateral Sclerosis Market Outlook
Amyotrophic lateral sclerosis (ALS) is a group of rare neurological diseases mainly involving the nerve cells (neurons) responsible for controlling voluntary muscle movement. Voluntary muscles produce movements like chewing, walking, and talking. The disease is progressive, meaning the symptoms get worse over time.
Currently, there is no cure for Amyotrophic Lateral Sclerosis and no effective treatment to halt or reverse the progression of the disease. The treatment landscape of Amyotrophic Lateral Sclerosis includes multidisciplinary care, such as physical therapy, speech therapy, dietary counselling, heat or whirlpool therapy and others. Medications are also prescribed to help manage symptoms of Amyotrophic Lateral Sclerosis, including pain, muscle cramps, stiffness, excess saliva and phlegm, and the pseudobulbar affect (involuntary or uncontrollable episodes of crying and/or laughing, or other emotional displays). Drugs also are available to help individuals with pain, depression, sleep disturbances, and constipation.
For Amyotrophic Lateral Sclerosis treatment, Riluzole is recommended as first-line therapy in all three regions – Japan, the US, and Europe. Riluzole was first approved in the US by the FDA in 1995, and it was later approved in many other countries in the ensuing decades. It is a medication that appears to prolong the life of some people with Amyotrophic Lateral Sclerosisby at least a few months. Riluzole seems to do two things: block sodium and calcium channels and increase glutamate clearance. Currently, no other drug is globally approved for slowing the progression of Amyotrophic Lateral Sclerosis. RILUTEK, TIGLUTIK, and EXSERVAN are brand names for different formulations of riluzole, a medicine used in the treatment of ALS. RILUTEK is an oral tablet, TIGLUTIK is an oral suspension, and EXSERVAN is an oral film. NEUDEXTA is approved for the treatment of pseudobulbar effects in conditions such as multiple sclerosis and ALS. Recently in June 2022, riluzole oral suspension TIGLUTIK was approved in China. In March 2022, Haisco Pharmaceutical acquired the exclusive rights to develop and commercialize EXSERVAN, for the treatment of Amyotrophic Lateral Sclerosis in China.
The most recent development in Amyotrophic Lateral Sclerosis was the addition of RELYVRIO for the treatment of Amyotrophic Lateral Sclerosis. On September 29, 2022, US FDA approved RELYVRIO (sodium phenylbutyrate and taurursodiol) for the treatment of adults with ALS. In May 2022, an oral suspension formulation of RADICAVA was approved by FDA for the treatment of Amyotrophic Lateral Sclerosis. The oral medication has the same dosing regimen as RADICAVA.
Nevertheless, recently some developmental initiatives have been taken towards the Amyotrophic Lateral Sclerosis treatment. Several pharmaceutical companies have taken to the initiative to meet the unmet needs of the present situation of the Amyotrophic Lateral Sclerosis market. Some of the key players are in the late and mid clinical development stages with their leading drug candidates.
The Amyotrophic Lateral Sclerosis pipeline possesses drugs in the late-stage development, many of which hold the potential to get launched in the forecast period. The key players includes Tofersen/BIIB067 (Biogen/Ionis Pharmaceuticals), ION363 (Ionis Pharmaceuticals), Ibudilast (MediciNova), Masitinib (AB Science), NurOwn (Brainstorm-Cell Therapeutics), Reldesemtiv (Cytokinetics), Gold Nanocrystals/CNM-Au8 (Clene Nanomedicine), Verdiperstat (Biohaven Pharmaceuticals), and others investigating their candidates for the treatment of Amyotrophic Lateral Sclerosis in the 7MM and China.
According to DelveInsight’s, Amyotrophic Lateral Sclerosis market size in 7MM and China is expected to witness a major change in the study period 2019-2032.
Key Findings
- The Amyotrophic Lateral Sclerosis market size is 7MM and China is expected to rise from USD 490 million in 2021 during the study period (2019–2032).
- Current market dynamics are dominated by the use of RILUTEK, TIGLUTIK, EXSERVAN, NEUDEXTA, RADICAVA, RADICAVA ORS, RELYVRIO, and others (Anti-epileptic drugs, Opioids, NSAIDs, Diuretics, SSRIs, Antidepressants, etc.)
- The therapies with the potential to get launched in the forecast period include Tofersen, ION363, NurOwn, Masitinib, and others. The launch of these therapies may increase market size in the coming years, assisted by an increasing patient pool of Amyotrophic Lateral Sclerosis patients.
- The United States accounts for the largest Amyotrophic Lateral Sclerosis market size compared to EU-4 (Germany, Italy, France, and Spain), the United Kingdom, Japan, and China
- In the United States, the Amyotrophic Lateral Sclerosis market size is anticipated to rise from USD 369 million in 2021 during the forecast period.
- Among the EU-4 countries, Germany had the largest Amyotrophic Lateral Sclerosis market size (USD 3 million) in 2021, while Spain had the smallest with USD 2 million.
- In 2021, Japan accounted for a market size of USD 44 million.
- In 2021, China accounted for a market size of USD 64 million
The United States: Amyotrophic Lateral Sclerosis Market Outlook
The total market size of Amyotrophic Lateral Sclerosis in the United States is expected to increase with a CAGR of 21.0% in the study period (2019–2032).
EU-4 (Germany, France, Italy, and Spain), the United Kingdom Countries: Market Outlook
The total market size of Amyotrophic Lateral Sclerosis in EU-4 is expected to increase during the study period (2019–2032).
Japan: Amyotrophic Lateral Sclerosis Market Outlook
The total market size of Amyotrophic Lateral Sclerosis in the Japan is expected to increase with a CAGR of 20.4% in the study period (2019–2032).
China: Amyotrophic Lateral Sclerosis Market Outlook
The total market size of Amyotrophic Lateral Sclerosis in the China is expected to increase with a CAGR of 14.4% in the study period (2019–2032).
Analyst Commentary
- The Amyotrophic Lateral Sclerosis pipeline is very robust, many potential therapies are being investigated for the treatment of ALS, and it is safe to predict that the treatment space will experience a significant impact on the market during the forecast period
- The European market has been quite uncertain about ALS drugs as Nuedexta was initially authorized by the EMA but later withdrawn at the request of the marketing authorization holder. Same scenario occured with Radicava, as Mitsubishi Tanabe Pharma notified the Committee for Medicinal Products for Human Use (CHMP) that it wishes to withdraw its application for a marketing authorisation for the treatment of ALS. So, these two therapies are currently not available in the Europe
- There is an ongoing HEALEY Amyotrophic Lateral Sclerosis platform trial, accelerating the path to new ALS therapies by testing multiple treatments at once, reducing the cost of research by 30%, decreasing the trial time by 50%, and increasing patient participation by 67%
- Most of the 7MM and China countries possess their own ALS registries, thereby providing a near precise country-wise number for diagnosed prevalent cases of the disease
- RADICAVA (edaravone) has been an effective therapy for ALS. Recently, oral RADICAVA got approved in the US for the treatment of patients with ALS to increase the patient compliance
Amyotrophic Lateral Sclerosis Drugs Uptake
This section focusses on the rate of uptake of the potential Amyotrophic Lateral Sclerosis drugs recently launched in the ALS market or expected to get launched in the market during the study period 2019-2032. The analysis covers ALS market uptake by drugs; patient uptake by therapies; and sales of each drug. For example-
Tofersen (Biogen/Ionis Pharmaceuticals), is an antisense drug designed to reduce the production of superoxide dismutase 1 (SOD1) is expected to first launch in US (2023) followed by EU-4 and UK (2024) Japan (2025), and China (2026). As per our analysis, Tofersen’s drug uptake in 7MM and China is expected to be fast in 7MM and medium- fast in China. Peak share is expected to be 0.9% US, EU-4 0.7%, UK (0.5%), Whereas in japan the peak share is higher approximately 2.6%, in China peak share is approximately 1.4%.
Note: Detailed emerging therapies assessment will be provided in the final report.
Amyotrophic Lateral Sclerosis Pipeline Development Activities
The Amyotrophic Lateral Sclerosis pipeline segment provides insights into different therapeutic candidates in Phase II and Phase III stage. It also analyses Amyotrophic Lateral Sclerosis companies involved in developing targeted therapeutics.
Development Activities
The Amyotrophic Lateral Sclerosis pipeline segment covers the detailed information of collaborations, acquisition and merger, licensing, patent details and other information for Amyotrophic Lateral Sclerosis emerging therapies.
Reimbursement Scenario
HealthWell Foundation is an independent non-profit organization providing financial assistance to adults and children to cover the cost of prescription drug coinsurance, copayments, deductibles, health insurance premiums and other selected out-of-pocket health care costs of ALS. It has an ALS fund that provides copay/premium as well as a pharmacy card fund. The treatments for ALS covered under the fund are Botox, Cuvposa, Edaravone, Elavil, Gabapentin, Glycopyrrolate, Increlex, Nuedexta, Radicava, Rilutek, Riluzole, and Tiglutik. The fund provides a maximum award level of USD 15,000 per year.
The Patient Advocate Foundation’s (PAF) Copay Relief (CPR) Program also has an ALS fund that provides copay, coinsurance and deductible. The fund has been developed in response to patients who have contacted PAF for help with their medication expenses and failed to receive it. The fund provides a maximum award level of USD 5,000 per year.
Besides these organizations, certain companies also provide financial assistance to ALS patients. For instance, ITF Pharma provides support and services for those impacted by ALS. The company’s Tiglutik copay support helps ensure that people get financial assistance in accessing the treatment they need. Eligible patients need not pay more than USD 50 per filled prescription of Tiglutik. The service is valid for each prescription fill for commercially insured patients where Tiglutik is covered.
Similarly, Mitsubishi Tanabe Pharma America has an out-of-pocket assistance program to support eligible patients with commercial insurance. Patients who have commercial insurance coverage for treatment with Radicava are eligible for enrollment into the program. With this program, eligible patients can save on the deductible, copay, and coinsurance costs for their medication and infusion costs for Radicava.
KOL- Views
To keep up with current market trends, we take KOLs and SME's opinion working in ALS domain through primary research to fill the data gaps and validate our secondary research. Some of the leaders are Principal Investigator and Center Director, Washington University School of Medicine; Professor of neurology, and Chairman, Department of Neurology, University Hospital and Medical Facility of Ulm; Principal Investigators and Professor of Neurology at Harvard Medical School and the Director of the Healey Center for ALS and Chair of Neurology at Mass General Hospital and others. Their opinion helps to understand and validate current and emerging therapies treatment patterns or ALS market trend. This will support the clients in potential upcoming novel treatment by identifying the overall scenario of the market and the Amyotrophic Lateral Sclerosis unmet needs.
Competitive Intelligence Analysis
We perform Competitive and Market Intelligence analysis of the ALS Market by using various Competitive Intelligence tools that include - SWOT analysis, PESTLE analysis, Porter's five forces, BCG Matrix, Market entry strategies etc. The inclusion of the analysis entirely depends upon the data availability.
Amyotrophic Lateral Sclerosis Market Forecast Report Scope
- The report covers the descriptive overview of Amyotrophic Lateral Sclerosis, explaining its causes, signs and symptoms, pathophysiology, diagnosis and currently available therapies
- Comprehensive insight has been provided into the Amyotrophic Lateral Sclerosis epidemiology and treatment in the 7MM and China
- Additionally, an all-inclusive account of both the current and emerging therapies for ALS are provided, along with the assessment of new therapies, which will have an impact on the current treatment landscape
- A detailed review of ALS market; historical and forecasted is included in the report, covering drug outreach in the 7MM and China
- The patient-based Amyotrophic Lateral Sclerosis market forecasting report provides an edge while developing business strategies, by understanding trends shaping and driving the global ALS market
Amyotrophic Lateral Sclerosis Market Research Report Highlights
- In the coming years, Amyotrophic Lateral Sclerosis market is set to change due to the rising awareness of the disease, and incremental healthcare spending across the world; which would expand the size of the market to enable the drug manufacturers to penetrate more into the market
- The Amyotrophic Lateral Sclerosis companies and academics are working to assess challenges and seek opportunities that could influence Amyotrophic Lateral Sclerosis R&D. The therapies under development are focused on novel approaches to treat/improve the disease condition
- Major players are involved in developing Amyotrophic Lateral Sclerosis therapies. Launch of emerging therapies will significantly impact the Amyotrophic Lateral Sclerosis market
- A better understanding of disease pathogenesis will also contribute to the development of novel therapeutics for Amyotrophic Lateral Sclerosis.
- Our in-depth analysis of the Amyotrophic Lateral Sclerosis pipeline assets across different stages of development (Phase III, Phase II, and Phase I/II), different emerging trends and comparative analysis of pipeline products with detailed clinical profiles, key cross-competition, launch date along with product development activities will support the clients in the decision-making process regarding their therapeutic portfolio by identifying the overall scenario of the research and development activities
Amyotrophic Lateral Sclerosis Market Forecast Report Insights
- Patient-based Amyotrophic Lateral Sclerosis Market Forecasting
- Therapeutic Approaches
- Amyotrophic Lateral Sclerosis Pipeline Analysis
- Amyotrophic Lateral Sclerosis Market Size
- Amyotrophic Lateral Sclerosis Market Trends
- Amyotrophic Lateral Sclerosis Market Opportunities
- Impact of Upcoming Therapies
Amyotrophic Lateral Sclerosis Market Forecast Report Key Strengths
- 10 Years Amyotrophic Lateral Sclerosis Market Forecast
- 7MM and China Coverage
- ALS Epidemiology Segmentation
- Key Cross Competition
- Highly Analyzed Market
- Amyotrophic Lateral Sclerosis Drugs Uptake
Amyotrophic Lateral Sclerosis Treatment Market Report Assessment
- Current Amyotrophic Lateral Sclerosis Treatment Market Practices
- Amyotrophic Lateral Sclerosis Unmet Needs
- Amyotrophic Lateral Sclerosis Pipeline Product Profiles
- Amyotrophic Lateral Sclerosis Market Attractiveness
- Amyotrophic Lateral Sclerosis Market Drivers
- Amyotrophic Lateral Sclerosis Market Barriers
Key Questions
Amyotrophic Lateral Sclerosis Treatment Market Insights:
- What was the ALS drug class share (%) distribution in 2019 and how it would look like in 2032?
- What would be the ALS total market size as well as market size by therapies across the 7MM and China during the study period (2023-2032)?
- What are the key findings pertaining to the market across 7MM and China and which country will have the largest ALS market size during the study period (2023-2032)?
- At what CAGR, the ALS market is expected to grow in 7MM and China during the study period (2023-2032)?
- What would be the ALS market outlook across the 7MM and China during the study period (2023-2032)?
- What would be the ALS market growth till 2032, and what will be the resultant market Size in the year 2032?
- How would the unmet needs affect the market dynamics and subsequent analysis of the associated trends?
Amyotrophic Lateral Sclerosis Epidemiology Insights:
- What is the disease risk, burden and regional/ethnic differences of Amyotrophic Lateral Sclerosis?
- What are the key factors driving the epidemiology trend for seven major markets covering the United States, EU-4 (Germany, Spain, France, and Italy), UK, Japan and China?
- What is the historical ALS patient pool in seven major markets covering the United States, EU-4 (Germany, Spain, France, and Italy), UK, Japan, and China?
- What would be the forecasted Amyotrophic Lateral Sclerosis patient pool in seven major markets covering the United States, EU-4 (Germany, Spain, France, and Italy), the UK, Japan, and China?
- Where will be the growth opportunities in the 7MM and China with respect to the patient population pertaining to Amyotrophic Lateral Sclerosis?
- Out of all 7MM and China countries, which country would have the highest prevalent population of Amyotrophic Lateral Sclerosis during the study period (2023-2032)?
- At what CAGR the patient population is expected to grow in 7MM and China during the study period (2023-2032)?
Current Amyotrophic Lateral Sclerosis Treatment Market Scenario, Marketed Drugs and Emerging Therapies:
- What are the current options for the ALS treatment in addition to the approved therapies?
- What are the current treatment guidelines for the treatment of ALS in the US, Europe, Japan and China?
- What are the ALS marketed drugs and their respective MOA, regulatory milestones, product development activities, advantages, disadvantages, safety and efficacy, etc.?
- How many companies are developing therapies for the treatment of ALS?
- How many therapies are in-development by each company for ALS treatment?
- How many are emerging therapies in mid-stage, and late stage of development for ALS treatment?
- What are the key collaborations (Industry - Industry, Industry - Academia), Mergers and acquisitions, licensing activities related to the ALS therapies?
- What are the recent novel therapies, targets, mechanisms of action and technologies being developed to overcome the limitation of existing therapies?
- What are the clinical studies going on for ALS and their status?
- What are the current challenges faced in drug development?
- What are the key designations that have been granted for the emerging therapies for ALS?
- What are the global historical and forecasted market of ALS?
Reasons to buy
- The patient-based Amyotrophic Lateral Sclerosis market forecasting report will help in developing business strategies by understanding trends shaping and driving the ALS market
- To understand the future market competition in the ALS market and Insightful review of the key market drivers and barriers
- Organize sales and marketing efforts by identifying the best opportunities for Amyotrophic Lateral Sclerosis in the US, EU-4 (Germany, Spain, Italy, and France), the United Kingdom, Japan, and China
- Identification of strong upcoming players in the market will help in devising strategies that will help in getting ahead of competitors
- Organize sales and marketing efforts by identifying the best opportunities for Amyotrophic Lateral Sclerosis market
- To understand the future market competition in the Amyotrophic Lateral Sclerosis market.
For More In-depth Information @ Latest DelveInsight Blog