Biliary Atresia Market Summary
- The Biliary Atresia Market is set for steady growth, with a robust compound annual growth rate (CAGR) anticipated from 2024 to 2034. The key drivers of the changing biliary atresia treatment market size in the US appear to be the rising incidence of the disease, advancements in diagnosis, increasing acceptance of treatments.
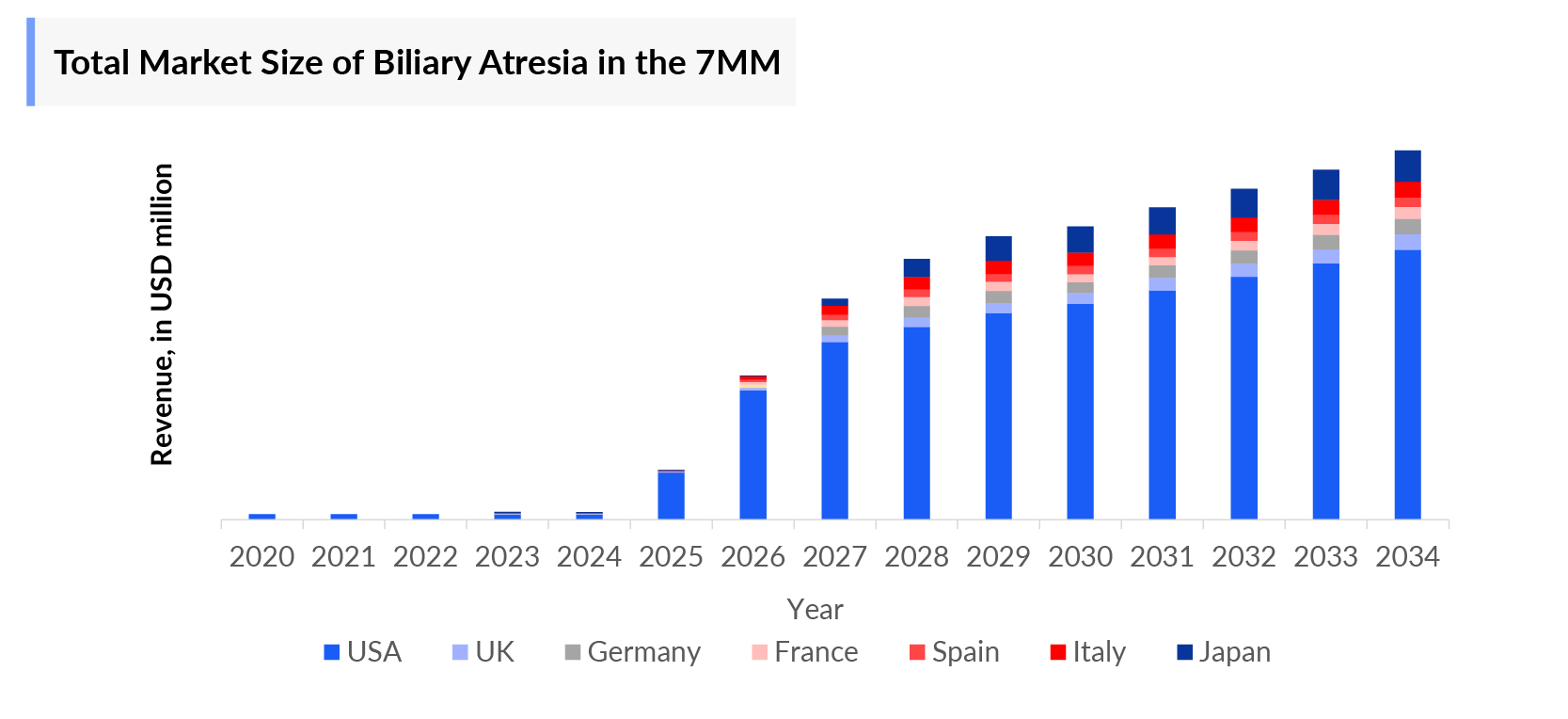
- Biliary Atresia Market is projected to continue growing significantly at a CAGR of 7.7%.
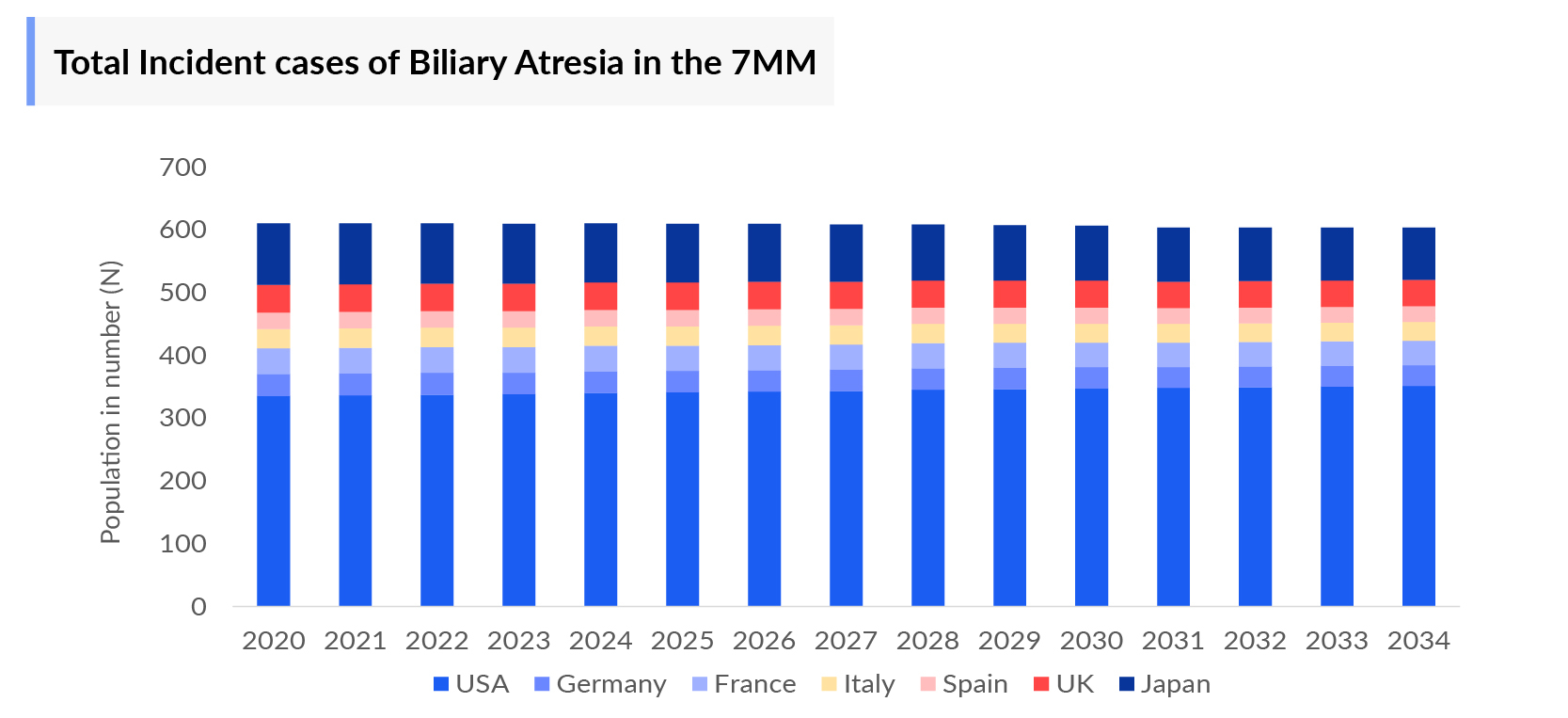
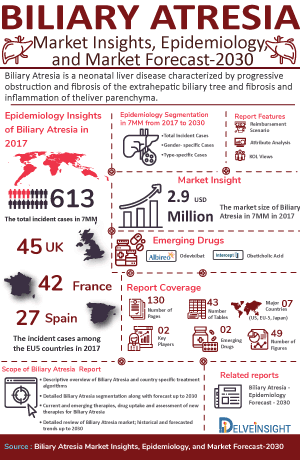
- According to DelveInsight’s estimates, in 2023, there were approximately 609 Biliary Atresia Incident Cases in the 7MM. Of these, the US accounted for nearly 56% of the cases, while EU4 and the UK, and Japan represented 28% and 16% of the cases, respectively.
Biliary Atresia Market Insights and Trends
- According to DelveInsight’s estimates, in 2023, there were approximately 609 Biliary Atresia Incident Cases in the 7MM. Of these, the US accounted for nearly 56% of the cases, while EU4 and the UK, and Japan represented 28% and 16% of the cases, respectively.
- Despite advancements, there remains a significant gap in biliary atresia treatment due to the lack of approved drug therapies, with current approaches mainly relying on surgical procedures like the Kasai operation and liver transplantation.
- Given the high Biliary Atresia unmet needs, several promising drugs are currently in development specifically targeting biliary atresia, including odevixibat, and obeticholic acid, among others.
Request for unlocking the CAGR of the Biliary Atresia Market
Factors Affecting Biliary Atresia Market Growth
Disease incidence and epidemiology
Biliary atresia is a rare neonatal disorder, but it remains the leading cause of pediatric liver transplantation. Variations in incidence across regions directly influence patient volume, healthcare demand, and overall Biliary Atresia market size.
Early diagnosis and newborn screening programs
Early detection significantly improves clinical outcomes, particularly when surgery is performed within the optimal time window. The adoption of newborn screening initiatives increases demand for diagnostic tools, specialist consultations, and early-stage interventions.
Standard treatment pathways and surgical outcomes
Kasai portoenterostomy is the primary initial treatment. Its success rate determines long-term disease management needs, including follow-up therapies and potential liver transplantation, shaping both short- and long-term market demand.
Limited but emerging therapeutic pipeline
The lack of approved disease-modifying pharmacological treatments limits market expansion; however, growing research activity and pipeline development in rare pediatric liver diseases present future growth opportunities.
Availability of specialized healthcare infrastructure
Access to pediatric hepatologists, neonatal surgeons, and transplant centers plays a critical role. Regions with advanced healthcare systems demonstrate higher treatment rates and stronger market penetration.
Reimbursement policies and orphan drug incentives
Supportive reimbursement frameworks and orphan disease incentives encourage investment in research and development. Conversely, reimbursement challenges can slow adoption of innovative therapies.
Advances in diagnostics and biomarkers
Improved diagnostic accuracy and faster confirmation of biliary atresia increase treatment initiation rates, enhancing demand for imaging, laboratory testing, and specialist care services.
Awareness among healthcare providers and caregivers
Increased awareness of prolonged neonatal jaundice and related symptoms improves referral rates, leading to earlier diagnosis and higher utilization of treatment services.
Improvements in surgical techniques and post-operative care
Advancements in surgical methods and post-operative management enhance survival outcomes, influencing demand for surgical devices, medications, and long-term disease management solutions.
Economic factors and public health priorities
Healthcare spending, public health policies, and prioritization of neonatal screening programs affect market accessibility and growth potential across different regions.
DelveInsight’s “Biliary atresia Market Insights, Epidemiology, and Market Forecast – 2034” report delivers an in-depth understanding of biliary atresia , historical and forecasted epidemiology, as well as the biliary atresia market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
The Biliary Atresia Market Size Report provides current treatment practices, emerging drugs, market share of individual therapies, and current and forecasted 7MM biliary atresia market size from 2020 to 2034. The report also covers biliary atresia treatment market practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s potential.
Scope of the Biliary Atresia Market Report | |
|
Study Period |
2020–2034 |
|
Forecast Period |
2024–2034 |
|
Geographies Covered |
|
|
Biliary Atresia Epidemiology |
|
|
Biliary Atresia Market |
|
|
Market Analysis |
|
|
Biliary Atresia Companies |
|
|
Future opportunity |
A key opportunity in the pharmacological treatment of biliary atresia lies in the development of innovative therapies targeting bile acid metabolism and cholestasis. Emerging agents, such as ileal bile acid transporter inhibitors like show promise in reducing serum bile acids and improving symptoms associated with cholestasis. Additionally, immunomodulatory treatments, including high-dose glucocorticoids, may enhance post-surgical outcomes. These advancements could significantly improve the quality of life for patients and reduce the need for liver transplantation, addressing a critical unmet need in the management of biliary atresia. |
Biliary Atresia Disease Understanding
Biliary atresia is a neonatal liver disease characterized by progressive obstruction and fibrosis of the extrahepatic biliary tree along with fibrosis and inflammation of the liver parenchyma. The etiology and pathogenesis of biliary atresia remain unknown. Recent studies are examining potential pathogenetic mechanisms of biliary atresia, including genetic susceptibility, the involvement of the immune system, and environmental insults such as viruses and toxins. However, it is possible that there is not a single etiological agent but rather a large group of injurious insults that might result in a final common pathway of extrahepatic bile duct obstruction and liver fibrosis.
The symptoms of biliary atresia usually appear by the age of 2–6 weeks. They include a yellowish coloration of the skin and whites of the eyes (jaundice), abnormally pale stools, and dark urine. Infants may also have swollen (distended) stomach and/or abnormal enlargement of the liver (hepatomegaly).
Biliary atresia Diagnosis
To diagnose biliary atresia, physicians begin with a thorough medical history and physical examination, focusing on symptoms like jaundice and changes in stool color. Blood tests assess liver function and bilirubin levels. An abdominal ultrasound evaluates the gallbladder's size and identifies potential abnormalities. If these tests suggest biliary atresia, a liver biopsy may be performed to analyze liver tissue. Definitive diagnosis often requires exploratory surgery to directly examine the bile ducts and confirm any blockages.
Further details related to country-based variations are provided in the report…
Biliary atresia Treatment
Management has been improved by public and professional education to encourage early referral and diagnosis to facilitate initial surgery before 8 weeks of age. Surgical management is complementary and includes an attempt to restore biliary flow (the Kasai portoenterostomy) and liver transplantation, if necessary. With the progress and advancement of transplantation surgery, liver transplantation is an option available to children who have either failed to restore the bile flow in the initial Kasai surgery or have developed advanced liver cirrhosis.
Medical management consists of antibiotics, ursodeoxycholic acid to encourage bile flow, fat-soluble vitamin supplementation, and nutritional support. It is a multifactorial disorder with a varied outcome depending upon the time of surgical treatment and histology.
Biliary Atresia Epidemiology
As the market is derived using a patient-based model, the biliary atresia epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by total incident cases of biliary atresia, type-specific cases of biliary atresia, gender-specific cases of biliary atresia, and treated cases of biliary atresia in the 7MM covering the United States, EU4 countries (Germany, France, Italy, and Spain) and the United Kingdom, and Japan from 2020 to 2034.
Key Findings from Biliary Atresia Epidemiological Analyses and Forecast
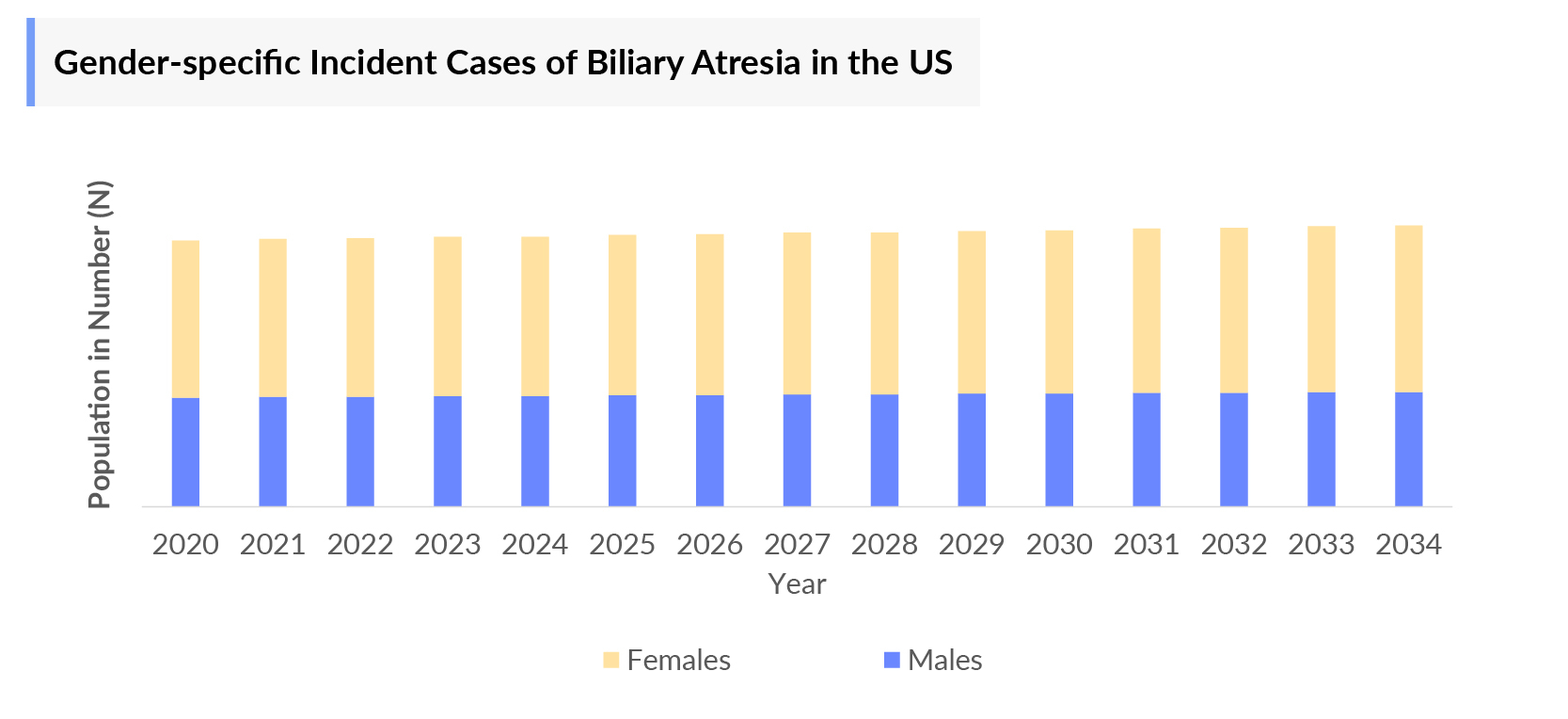
- According to DelveInsight’s epidemiology model, in 2023, the Incident cases of biliary atresia in the US was found to be approximately 338, these case are expected to increase by 2034.
- In 2023, there were nearly 17 cases of Type I, 7 cases of Type II and 315 cases for Type III. Our estimates suggest that these cases will change during the forecast period (2024-2034).
- In 2023, Germany saw approximately 34 cases of biliary atresia, with around 17 cases in males and 18 in females.
- In 2023, in the UK there were approximately 19 cases of biliary atresia in males and 25 cases in females. Assessments as per DelveInsight’s analysts show that the overall cases of biliary atresia in both the genders is subjected to decrease in the coming years directly attributed to the country population that is decreasing by 2034, in males and females, respectively.
- In 2023, in Japan there were 11 cases of Type I and 2 cases of Type II. Assessments as per DelveInsight’s analysts show that the overall incidence of biliary atresia is maximum for type III with 80 cases.
Unlock comprehensive insights! Click Here to Purchase the Full Epidemiology Report @ Biliary Atresia Incidence
Biliary Atresia Drugs Analysis
Biliary Atresia Emerging Drugs
BYLVAY (odevixibat): Ipsen
Odevixibat (A-4250), a potent and selective inhibitor of the ileal bile acid transporter (IBAT), also known as apical sodium-dependent bile acid transporter (ASBT), acts locally in the gut, and has minimal systemic exposure at the therapeutic dose. IBAT initiates the transport of bile acids, which flow through the portal vein back to the liver in a process known as enterohepatic circulation. Approximately 95% of bile acids are recirculated via the IBAT to the liver. Accordingly, a product capable of inhibiting the IBAT could lead to a reduction in bile acids returning to the liver and may represent a promising approach for treating cholestatic liver diseases. Odevixibat is being developed by Ipsen that completed acquisition of Albireo, expanding the scope of its rare disease portfolio.
Currently a Phase III trial is ongoing to assess efficacy and safety of odevixibat in children with biliary atresia who have undergone a Kasai HPE. Odevixibat has been granted an orphan drug designations the US and EU for treating biliary atresia. Moreover, the drug candidate is approved for patients living with cholestatic pruritus due to Alagille syndrome.
Obeticholic Acid: Intercept Pharmaceuticals
Obeticholic acid (OCA) is a first-in-class agonist that selectively binds to the farnesoid X receptor (FXR), a nuclear receptor expressed in the liver and intestine. FXR is a key regulator of bile acid, inflammatory, fibrotic, and metabolic pathways. FXR activation decreases the intracellular hepatocyte concentrations of bile acids by suppressing de novo synthesis from cholesterol as well as by increased transport of bile acids out of the hepatocytes. These mechanisms limit the overall size of the circulating bile acid pool while promoting choleresis, thus reducing hepatic exposure to bile acids.
The company is currently in Phase II/III trial to assess efficacy, safety, tolerability, pharmacokinetics, and pharmacodynamics of obeticholic acid compared to placebo in pediatric participants with biliary atresia.
Get More Insights of the Report @ Obeticholic Acid Market
|
Drug |
MoA |
RoA |
Company |
Phase |
|
BYLVAY (odevixibat) |
Selective inhibitor of ileal bile acid transporter (IBAT) |
Oral |
Ipsen |
III |
|
Obeticholic acid |
Farnesoid X receptor (FXR) agonist |
Oral |
Intercept Pharmaceuticals |
II/III |
Biliary Atresia Drugs Market Insights
In the postoperative management, medical care following hepatoportoenterostomy consists of interventions such as choleretics and possible use of anti-inflammatory medications, nutritional rehabilitation, fat-soluble vitamin supplementation, prevention of cholangitis, management of portal hypertension and its sequel. Long-term antibiotic prophylaxis, such as trimethoprim-sulfamethoxazole, is often prescribed to reduce the risk of cholangitis, an infection of the bile ducts that can occur post-surgery. While there is no conclusive evidence supporting its efficacy, it is commonly used in clinical practice.
Ursodeoxycholic acid may enhance bile flow in patients with a patent biliary system after the Kasai procedure. It has been shown to improve outcomes in some infants, although its long-term safety profile requires careful monitoring due to potential adverse effects observed in other conditions.
Biliary Atresia Market Outlook
Biliary Atresia is characterized by a fibro proliferative obliteration of the biliary tree that progresses toward hepatic fibrosis, cirrhosis, and end-stage liver failure. The Biliary Atresia treatment is surgical and currently recommended as a sequence of, eventually, two interventions. During the first months of life, a Hepatoportoenterostomy (a “Kasai,” modifications) should be performed, to restore the biliary flow to the intestine and lessen further damage to the liver. If this fails or the disease progresses towards biliary cirrhosis and life-threatening complications, then liver transplantation is indicated, for which biliary atresia represents the most frequent pediatric indication.
In the Postoperative Management, medical care following HPE consists of interventions such as Choleretics and possible use of anti-inflammatory medications, Nutritional rehabilitation, Fat-soluble vitamin supplementation, Prevention of cholangitis, management of portal hypertension and its sequel. Administration of choleretics such as ursodeoxycholic acid (UDCA) is standard practice in BA, although its clinical utility has not been definitively established. Given by mouth, this hydrophilic bile acid shifts the balance of bile acids toward hydrophilic forms. This is thought to stabilize membranes and reduce the generation of free radicals, thus protecting mitochondria from damage.
High-energy supplements, such as glucose polymers or medium-chain triglyceride oil, are used to fortify formula or solid foods. MCT oil / MCT based formula is useful because it is calorically rich, and patients with cholestasis readily absorb it because it does not require micellar solubilization. The treatment of biliary atresia is multifaceted, focusing on identifying and addressing the unde rlying cause while managing symptoms to improve patient comfort.
Biliary Atresia Drugs Uptake
This section focuses on the uptake rate of potential Biliary Atresia drugs expected to be launched in the Biliary Atresia market during 2020–2034.
Biliary Atresia Clinical Trial Activities
The Biliary Atresia pipeline segment report provides insights into different Biliary Atresia clinical trials within Phase III, Phase II, and Phase I. It also analyzes key Biliary Atresia Companies involved in developing targeted therapeutics.
Biliary Atresia Pipeline development activities
The Biliary Atresia pipeline segment report covers information on collaborations, acquisitions and mergers, licensing, and patent details for Biliary Atresia emerging therapies.
Take Your Research to the Next Level! Click Here to Get Access to the Full Pipeline Report @ Biliary Atresia Treatment Drugs
Latest KOL Views on Biliary Atresia
To keep up with current Biliary Atresia market trends, we take KOLs and SMEs’ opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts contacted for insights on biliary atresia evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake, along with challenges related to accessibility, including Medical/scientific writers, Medical Professionals, Professors, Directors, and Others.
DelveInsight’s analysts connected with 50+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. Centers like Hepatology and Nutrition at Cincinnati Children's, Children's Hospital Colorado, Pediatric Hepatologist at Children’s Healthcare of Atlanta among others were contacted. Their opinion helps understand and validate current and emerging therapy treatment patterns or biliary atresia market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the Biliary Atresia market and the unmet needs.
Physician’s View on Biliary Atresia
Biliary atresia is generally viewed as a challenging condition due to its rarity and the limited treatment options available. The standard approach often involves early surgical intervention, such as the Kasai procedure, to improve bile flow, but the success of this surgery varies, and many patients ultimately require liver transplantation. Physicians emphasize the need for earlier diagnosis and more effective pharmacological therapies to improve long-term outcomes and reduce the dependency on invasive procedures.
“As per a KOL in the US, despite the association of young age with treatment response, age alone does not predict clinical outcome nor correlates reliably with liver histological scoring or transcriptional profile. With substantial variability in clinical course, the discovery of predictive biomarkers at the time of diagnosis would be invaluable to the field by enabling the customization of treatment protocols and the stratification of patients into clinical trials.” “According to another KOL, in some patients, fibrosis may represent a later stage of liver disease that may not be uniformly correlated with the age at diagnosis, or it may represent a rapid fibrosis program that is poorly understood and limited to a subgroup of patients.”
Biliary Atresia Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Conjoint Analysis analyzes multiple emerging Biliary Atresia therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy. To analyze the effectiveness of these therapies, have calculated their attributed analysis by giving them scores based on their ability to improve atrial and ventricular dimension/function and ability to regulate heart rate.
Further, the therapies’ safety is evaluated wherein the adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials, which directly affects the safety of the molecule in the upcoming trials. It sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the route of administration, order of entry and designation, probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Biliary Atresia Treatment Market Report Scope
- The Biliary Atresia treatment maarket report covers a segment of key events, an executive summary, and a descriptive overview of biliary atresia explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, disease progression, and treatment guidelines have been provided.
- Additionally, an all-inclusive account of the current and emerging therapies and the elaborative profiles of late-stage and prominent therapies will impact the current treatment landscape.
- A detailed review of the biliary atresia market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The Biliary Atresia Treatment Market Report provides an edge while developing business strategies by understanding trends through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help shape and drive the 7MM Biliary Atresia Drugs Market.
Biliary Atresia Market Report Insights
- Patient-based Biliary Atresia Market Forecasting
- Biliary Atresia Therapeutic Market Approaches
- Biliary Atresia Pipeline Analysis
- Biliary Atresia Market Size
- Biliary Atresia Market Trends
- Existing and Future Biliary Atresia Market Opportunity
Biliary Atresia Market Report Key Strengths
- 11 years Biliary Atresia Market Forecast
- The 7MM Coverage
- Biliary atresia Epidemiology Segmentation
- Key Cross Competition
- Attribute analysis
- Biliary Atresia Drugs Uptake
- Key Biliary Atresia Market Forecast Assumptions
Biliary Atresia Market Report Assessment
- Current Biliary Atresia Treatment Market Practices
- Biliary Atresia Unmet Needs
- Biliary Atresia Pipeline Product Profiles
- Biliary Atresia Drugs Market Attractiveness
- Qualitative Analysis (SWOT and Attribute Analysis)
- Biliary Atresia Market Drivers
- Biliary Atresia Market Barriers
Key Questions Answered in the Biliary Atresia Market Report
Biliary Atresia Treatment Market Insights
- What was the total Biliary Atresia Market Size, the Biliary Atresia Market Size by therapies, and Biliary Atresia market share (%) distribution in 2020, and what would it look like by 2034? What are the contributing factors for this growth?
- How will BYLVAY (odevixibat) affect the treatment paradigm of biliary atresia?
- How will Obeticholic Acid compete with the upcoming therapies?
- Which Biliary Atresia drug is going to be the largest contributor by 2034?
- What are the pricing variations among different geographies for approved and marketed therapies?
- How would future opportunities affect the market dynamics and subsequent analysis of the associated trends?
Biliary Atresia Epidemiology Insights
- What are the disease risks, burdens, and unmet needs of biliary atresia? What will be the growth opportunities across the 7MM with respect to the patient population pertaining to biliary atresia?
- What is the historical and forecasted biliary atresia patient pool in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan?
- Out of the countries mentioned above, which country would have the highest diagnosed prevalent biliary atresia population during the forecast period (2024–2034)?
- What factors are contributing to the growth of biliary atresia cases?
Current Biliary Atresia Treatment Market Scenario, Marketed Drugs, and Emerging Therapies
- What are the current options for the Biliary Atresia Treatment? What are the current clinical and treatment guidelines for treating biliary atresia?
- How many companies are developing therapies for the Biliary Atresia Treatment?
- How many emerging therapies are in the mid-stage and late stage of development for treating biliary atresia?
- What are the recent novel therapies, targets, mechanisms of action, and technologies developed to overcome the limitations of existing therapies?
- What is the cost burden of current treatment on the patient?
- Patient acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the accessibility issues of approved therapy in the US?
- What is the 7MM historical and forecasted Biliary Atresia Market?
Reasons to Buy Biliary Atresia Drugs Market Report
- The Biliary Atresia Treatment Market Report will help develop business strategies by understanding the latest trends and changing treatment dynamics driving the biliary atresia drugs market.
- Insights on patient burden/disease Biliary Atresia prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- Understand the existing Biliary Atresia market opportunities in varying geographies and the growth potential over the coming years.
- The distribution of historical and current patient share is based on real-world prescription data in the US, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
- Identifying upcoming Biliary Atresia Companies in the Biliary Atresia drugs market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the conjoint analysis section to provide visibility around leading classes.
- Highlights of Access and Reimbursement policies for biliary atresia, barriers to accessibility of approved therapy, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing Biliary Atresia market so that the upcoming Biliary Atresia players can strengthen their development and launch strategy.
Stay Updated with us for Recent Articles:-