Cryopyrin-Associated Periodic Syndrome Market
Key Highlights
- Cryopyrin-associated Periodic Syndromes (CAPS) is a rare group of autoinflammatory disorders characterized by autosomal dominant inheritance. These conditions are believed to be linked with mutations in exon 3 of the NLRP3 gene, responsible for encoding the Cryopyrin protein.
- CAPS comprises a group of three disorders, varying with their severity and the organs they tend to affect upon the onset, namely Neonatal Onset Multisystem Inflammatory Disease (NOMID), also called Chronic Inflammatory Neurological Cutaneous Articular Syndrome (CINCA), Familial cold auto-inflammatory syndrome (FCAS) and Muckle-Wells syndrome (MWS).
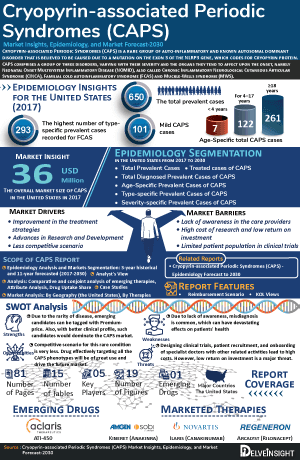
- As per DelveInsight estimates, In 2023, the United States reported app~120 cases of mild CAPS, ~300 cases of moderate CAPS, and ~60 cases of severe CAPS. These numbers are projected to increase in the forecasted period.
- KINERET, ILARIS, ARCALYST, and others are approved for the treatment of CAPS.
- The market is anticipated to witness a substantial positive shift owing to better uptake of existing drugs, the expected market launch of therapies, and raised awareness.
- The United States accounts for the largest market size of Cryopyrin-associated Periodic Syndromes (CAPS), in comparison to EU4 (Germany, Spain, Italy, France), the United Kingdom, and Japan.
- CAPS pipeline is not so robust but possesses potential drugs such as VTX2735.
- In early 2024, Ventyx Biosciences announced positive Phase II trial results for VTX2735 in patients with cryopyrin-associated periodic syndromes (CAPS). The treatment showed significant improvements in disease activity and reductions in key inflammatory biomarkers such as hsCRP, SAA, and IL-6.
DelveInsight's “Cryopyrin-associated Periodic Syndromes (CAPS) – Market Insights, Epidemiology and Market Forecast – 2034” report delivers an in-depth understanding of Cryopyrin-associated Periodic Syndromes (CAPS), historical and forecasted epidemiology as well as the Cryopyrin-associated Periodic Syndromes (CAPS) market trends in the United States, EU4 (Germany, Spain, Italy, and France) and the United Kingdom, and Japan.
Cryopyrin-associated Periodic Syndromes (CAPS) market report provides real-world prescription pattern analysis, emerging drugs, market share of individual therapies, and historical and forecasted 7MM Cryopyrin-associated Periodic Syndromes (CAPS) market size from 2020 to 2034. The report also covers current Cryopyrin-associated Periodic Syndromes (CAPS) treatment practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s underlying potential.
Geography Covered
- The United States
- EU4 (Germany, France, Italy, and Spain) and the United Kingdom
- Japan
|
Study Period |
2020–2034 |
|
Forecast Period |
2024–2034 |
|
Geographies Covered |
US, EU4 (Germany, France, Italy, and Spain) the UK, and Japan |
|
Cryopyrin-associated Periodic Syndromes (CAPS) Epidemiology |
Segmented by:
|
|
Cryopyrin-associated Periodic Syndromes (CAPS) key companies |
|
|
Cryopyrin-associated Periodic Syndromes (CAPS) key therapies/drug |
|
|
Cryopyrin-associated Periodic Syndromes (CAPS) Market |
Segmented by:
|
|
Analysis |
|
Cryopyrin-associated Periodic Syndromes (CAPS) Understanding and Treatment Algorithm
Cryopyrin-associated Periodic Syndromes (CAPS) Overview, Country-Specific Treatment Guidelines and Diagnosis
Cryopyrin-Associated Periodic Syndromes (CAPS) are a group of rare autoinflammatory disorders caused by mutations in the NLRP3 gene, which leads to excessive production of interleukin-1β. This results in recurrent episodes of systemic inflammation. CAPS includes three main syndromes: Familial Cold Autoinflammatory Syndrome (FCAS), Muckle-Wells Syndrome (MWS), and Neonatal-Onset Multisystem Inflammatory Disease (NOMID). Symptoms can vary widely but commonly include recurrent fever, rash, joint pain, and in severe cases, neurological complications. The clinical presentation can overlap among the syndromes, making diagnosis challenging. Early recognition is crucial to manage symptoms effectively and prevent long-term complications such as hearing loss and amyloidosis.
Diagnosing CAPS involves a comprehensive approach that includes a detailed patient history, physical examination, and genetic testing for mutations in the NLRP3 gene. Clinicians look for characteristic symptoms such as recurrent fevers, rashes, and joint pain, particularly in response to cold exposure in cases of FCAS. Laboratory tests may reveal elevated inflammatory markers like C-reactive protein (CRP) and serum amyloid A (SAA) during flares. The use of standardized tools, such as the autoinflammatory disease activity index (AIDAI), can help track symptoms and disease activity. Due to the heterogeneous nature of CAPS, a multidisciplinary approach is often necessary to ensure accurate diagnosis and effective management.
Further details related to country-based variations in diagnosis are provided in the report…
Cryopyrin-associated Periodic Syndromes (CAPS) Treatment
The treatment of CAPS primarily focuses on controlling inflammation and preventing complications. Targeted therapies that inhibit interleukin-1, such as canakinumab (a monoclonal antibody) and anakinra (an IL-1 receptor antagonist), are the mainstay of treatment. These medications can significantly reduce the frequency and severity of inflammatory episodes. Rilonacept, another IL-1 inhibitor is also used in some cases. Early initiation of these therapies is crucial to minimize the risk of organ damage and improve the quality of life for patients. Additionally, a multidisciplinary management strategy, including regular monitoring of disease activity and psychosocial support, is essential for optimal patient care.
Cryopyrin-associated Periodic Syndromes (CAPS) Epidemiology
The Cryopyrin-associated Periodic Syndromes (CAPS) epidemiology chapter in the report provides historical as well as forecasted in the 7MM covering the United States, EU4 countries (Germany, France, Italy, and Spain), the United Kingdom, and Japan from 2024 to 2034. The Cryopyrin-associated Periodic Syndromes (CAPS) epidemiology is segmented with detailed insights into Total Prevalent Cases, Total Diagnosed Prevalent Cases, Age-Specific Cases, Type-specific, Severity-specific, and Treated cases of Cryopyrin-associated Periodic Syndromes (CAPS).
- In 2023, the total prevalent cases in the United States were approximately 700, which is anticipated to increase in the forecasted period.
- In 2023, there were approximately 500 diagnosed cases of CAPS in the United States.
- In 2023, among the total CAPS cases in the United States, approximately 10 cases were in individuals under 4 years old, about 150 cases were in those aged 4–17 years, and around 300 cases were in individuals aged 18 years and older.
Cryopyrin-associated Periodic Syndromes (CAPS) Drug Chapter
The drug chapter segment of the Cryopyrin-associated Periodic Syndromes (CAPS) report encloses a detailed analysis of Cryopyrin-associated Periodic Syndromes (CAPS) marketed drugs and late-stage (Phase III and Phase II) pipeline drugs. It also deep dives into the Cryopyrin-associated Periodic Syndromes (CAPS) pivotal clinical trial details, recent and expected market approvals, patent details, the latest news, and recent deals and collaborations.
Cryopyrin-associated Periodic Syndromes (CAPS) Marketed Drugs
KINERET (Anakinra): Amgen/Biovitrum
Kineret (anakinra) a very renowned Amgen product, is a recombinant, non-glycosylated form of the human interleukin-1 receptor antagonist (IL-1Ra). It is approved by the FDA for the treatment of Neonatal-Onset Multisystem Inflammatory Disease (NOMID). The recommended starting dose of Kineret is 1-2 mg/kg for NOMID patients. The dose can be individually adjusted to a maximum of 8 mg/kg daily to control active inflammation. Adjust doses in 0.5 to 1.0 mg/kg increments. Once daily administration is generally recommended, but the dose may be split into twice daily administrations. Each syringe is intended for a single use. A new syringe must be used for each dose. Any unused portion after each dose should be discarded.
Kineret blocks the biological activity of IL-1 alpha and beta by competitively inhibiting IL-1 binding to the interleukin-1 type I receptor (IL-1RI), which is expressed in a wide variety of tissues and organs. IL-1 production is induced in response to inflammatory stimuli and mediates various physiologic responses including inflammatory and immunological responses. Spontaneous mutations in the CIAS1/NLRP3 gene have been identified in a majority of patients with CAPS such as NOMID. CIAS1/NLRP3 encodes for cryopyrin, a component of the inflammasome. The activated inflammasome results in proteolytic maturation and secretion of IL-1β, which has an important role in the systemic inflammation and manifestations of NOMID.
ILARIS (Canakinumab): Novartis
Ilaris (Canakinumab), previously known as ACZ885, is a recombinant, human anti-human-IL-1β monoclonal antibody that belongs to the IgG1/κ isotype subclass. It is expressed in a murine Sp2/0-Ag14 cell line and comprised of two 447- (or 448) residue heavy chains and two 214-residue light chains. The recommended dose of ILARIS is 150 mg every eight weeks for CAPS patients with body weight greater than 40 kg. For CAPS patients with body weight greater than or equal to 15 kg and less than or equal to 40 kg, the recommended dose is 2 mg/kg every eight weeks. For children 15 to 40 kg with an inadequate response, the dose can be increased to 3 mg/kg. ILARIS is administered every eight weeks.
Canakinumab binds to human IL1β and neutralizes its activity by blocking its interaction with IL-1 receptors, but it does not bind IL-1α or IL-1 receptor antagonist (IL-1ra). Mutations in NLRP-3 result in an overactive inflammasome resulting in excessive release of activated IL-1β that drives inflammation. Still’s disease is a severe auto-inflammatory disease, driven by innate immunity utilizing proinflammatory cytokines such as IL-1β.
Note: Detailed current therapies assessment will be provided in the full report of Cryopyrin-associated Periodic Syndromes (CAPS)…
Cryopyrin-associated Periodic Syndromes (CAPS) Emerging Drugs
VTX2735: Ventyx Biosciences
VTX2735 is an oral, selective inhibitor of NLRP3 that is designed for the treatment of systemic inflammatory conditions, with an initial focus on cardiovascular diseases. NLRP3 targets the inflammasome/IL-1β axis to break the inflammation feed-forward loop in autoinflammatory and autoimmune diseases
Inflammasome inhibition offers a compelling therapeutic pathway for inflammatory disorders with high unmet needs. Inflammasomes are multi-protein complexes that sense molecular hallmarks of infection or cellular injury and initiate an appropriate immune response. NLRP3, the best-characterized inflammasome, is known to be activated by a range of non-infectious tissue damage signals associated with aging, physical inactivity, and obesity. Activated NLRP3 initiates immune responses, stimulating the production of inflammatory cytokines (IL-1β and IL-18) as well as a type of cell death called pyroptosis and further tissue damage.
In early 2024, Ventyx Biosciences announced positive Phase II trial results for VTX2735 in patients with cryopyrin-associated periodic syndromes (CAPS). The treatment showed significant improvements in disease activity and reductions in key inflammatory biomarkers such as hsCRP, SAA, and IL-6.
Note: Detailed emerging therapies assessment will be provided in the final report…
Cryopyrin-associated Periodic Syndromes (CAPS) Market Outlook
- Key players, such as Ventyx Biosciences, and others are evaluating their lead candidates in different stages of clinical development, respectively. They aim to investigate their products for the treatment of Cryopyrin-associated Periodic Syndromes (CAPS).
- In 2023, the market size of the United States was accessed to be approximately USD 40 million. With the expected launch of the upcoming therapy, the overall market size of CAPS will experience an increase throughout the study period [2020–2034].
- Among the approved therapies in the United States, Canakinumab generated the highest revenue approximately USD 25 million in 2023.
- Apart from this, NSAIDS and Corticosteroids are used to lower elevated temperatures and to provide relief for inflamed areas of the body.
Cryopyrin-associated Periodic Syndromes (CAPS) Drug Uptake
This section focuses on the uptake rate of potential drugs expected to be launched in the market during 2024–2034, which depends on the competitive landscape, safety, and efficacy data along with order of entry. It is important to understand that the key players evaluating their novel therapies in the pivotal and confirmatory trials should remain vigilant when selecting appropriate comparators to stand the greatest chance of a positive opinion from regulatory bodies, leading to approval, smooth launch, and rapid uptake.
Further detailed analysis of emerging therapies drug uptake in the report…
Cryopyrin-associated Periodic Syndromes (CAPS) Activities
The report provides insights into different therapeutic candidates in Phase III and Phase II stages. It also analyzes key players involved in developing targeted therapeutics.
Pipeline Development Activities
The report covers information on collaborations, acquisitions and mergers, licensing, and patent details for Cryopyrin-associated Periodic Syndromes (CAPS) emerging therapies.
KOL Views
To keep up with the real-world scenario in current and emerging market trends, we take opinions from Key Industry leaders working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts were contacted for insights on the evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake along with challenges related to accessibility.
DelveInsight’s analysts connected with 20+ KOLs to gather insights; however, interviews were conducted with 9+ KOLs in the 7MM. Their opinion helps understand and validate current and emerging treatment patterns of Cryopyrin-associated Periodic Syndromes (CAPS). This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT analysis and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of gaps in disease diagnosis, patient awareness, physician acceptability, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided.
Conjoint Analysis analyzes multiple approved and emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
In efficacy, the trial’s primary and secondary outcome measures are evaluated; for instance one of the most important primary outcome measures is safety and tolerability of the drug.
Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Market Access and Reimbursement
The report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of currently used therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Scope of the Cryopyrin-associated Periodic Syndromes (CAPS) Market Report
- The report covers a segment of key events, an executive summary, descriptive overview of Cryopyrin-associated Periodic Syndromes (CAPS), explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight has been provided into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, and disease progression along with country specific treatment guidelines.
- Additionally, an all-inclusive account of both the current and emerging therapies, along with the elaborative profiles of late-stage and prominent therapies, will have an impact on the current treatment landscape.
- A detailed review of the Cryopyrin-associated Periodic Syndromes (CAPS) market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help in shaping and driving the 7MM Cryopyrin-associated Periodic Syndromes (CAPS) market.
Cryopyrin-associated Periodic Syndromes (CAPS) Market Report Insights
- Patient Population
- Therapeutic Approaches
- Cryopyrin-associated Periodic Syndromes (CAPS) Pipeline Analysis
- Cryopyrin-associated Periodic Syndromes (CAPS) Market Size and Trends
- Existing and future Market Opportunity
Cryopyrin-associated Periodic Syndromes (CAPS) Market Report Key Strengths
- Eleven Years Forecast
- 7MM Coverage
- Cryopyrin-associated Periodic Syndromes (CAPS) Epidemiology Segmentation
- Inclusion of Country specific treatment guidelines
- KOL’s feedback on approved and emerging therapies
- Key Cross Competition
- Conjoint analysis
- Drugs Uptake and Key Market Forecast Assumptions
Cryopyrin-associated Periodic Syndromes (CAPS) Market Report Assessment
- Current Treatment Practices
- Unmet Needs
- Pipeline Product Profiles
- Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
FAQs
- What is the growth rate of the 7MM Cryopyrin-associated Periodic Syndromes (CAPS) treatment market?
- What was the Cryopyrin-associated Periodic Syndromes (CAPS) total market size, the market size by therapies, market share (%) distribution in 2020, and what would it look like in 2034? What are the contributing factors/key catalysts for this growth?
- Is there any unexplored patient setting that can open the window for growth in the future?
- What are the pricing variations among different geographies for approved and off-label therapies?
- How would the market drivers, barriers, and future opportunities affect the market dynamics and subsequent analysis of the associated trends? Although multiple expert guidelines recommend testing for targetable mutations before therapy initiation, why do barriers to testing remain high?
- What are the current and emerging options for the treatment of Cryopyrin-associated Periodic Syndromes (CAPS)?
- How many companies are developing therapies for the treatment of Cryopyrin-associated Periodic Syndromes (CAPS)?
- What are the recent novel therapies, targets, mechanisms of action, and technologies developed to overcome the limitations of existing therapies?
- Patient/physician acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the country-specific accessibility issues of expensive, recently approved therapies?
Reasons to buy
- The report will help in developing business strategies by understanding the latest trends and changing treatment dynamics driving the Cryopyrin-associated Periodic Syndromes (CAPS) Market.
- Insights on patient burden/disease prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years
- Understand the existing market opportunities in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identifying strong upcoming players in the market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the conjoint analysis section to provide visibility around leading classes.
- Highlights of access and reimbursement policies of approved therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing market so that the upcoming players can strengthen their development and launch strategy.