Glioblastoma Market
- As per DelveInsight, the Glioblastoma Market is expected to expand at a healthy growth rate during the forecast period (2024-2034), owing to the launch of new therapies in the market and the rise in the number of cases.
- The leading Glioblastoma Companies such as Bayer, Chimerix, Aivita Biomedical, Denovo Biopharma, Northwest Therapeutics, VBL Therapeutics, Laminar Pharmaceuticals, MedImmune, DNAtrix, Immunomic Therapeutics, Imvax, MimiVax, CNS Pharmaceuticals, Epitopoietic Research Corporation (ERC), Istari Oncology, SonALAsense, Kintara Therapeutics, Bristol Myers Squibb, Medicenna Therapeutics, BioMimetix, Eisai, Merck Sharp & Dohme, Kazia Therapeutics, Oblato, Genenta Science, Enterome, Inovio Pharmaceuticals, Karyopharm Therapeutics, Forma Therapeutics, VBI Vaccines, and TME Pharma., and others.
Download the Sample PDF to Get More Insight @ Glioblastoma Treatment Market
DelveInsight's "Glioblastoma Market Insights, Epidemiology and Market Forecast– 2034" report delivers an in-depth understanding of the Glioblastoma, historical and forecasted epidemiology as well as the Glioblastoma market trends in the United States, the EU-4 (Germany, Spain, Italy, and France), the United Kingdom, and Japan.
The Glioblastoma Treatment Market Report provides current treatment practices, emerging drugs, and market share of individual therapies, with current and forecasted 7MM market size from 2020 to 2034. The report also covers current Glioblastoma treatment market practice/algorithm, and Glioblastoma unmet needs to curate the best of the opportunities and assesses the underlying potential of the market.
|
Study Period |
2020 to 2034 |
|
Forecast Period |
2024-2034 |
|
Geographies Covered |
|
|
Glioblastoma Treatment Market |
|
|
Glioblastoma Treatment Market Size | |
|
Glioblastoma Companies |
Bayer, Chimerix, Aivita Biomedical, Denovo Biopharma, Northwest Therapeutics, VBL Therapeutics, Laminar Pharmaceuticals, MedImmune, DNAtrix, Immunomic Therapeutics, Imvax, MimiVax, CNS Pharmaceuticals, Epitopoietic Research Corporation (ERC), Istari Oncology, SonALAsense, Kintara Therapeutics, Bristol Myers Squibb, Medicenna Therapeutics, BioMimetix, Eisai, Merck Sharp & Dohme, Kazia Therapeutics, Oblato, Genenta Science, Enterome, Inovio Pharmaceuticals, Karyopharm Therapeutics, Forma Therapeutics, VBI Vaccines, and TME Pharma, and others. |
|
Glioblastoma Epidemiology Segmentation |
|
Glioblastoma Multiforme Treatment Market
Glioblastoma is the most frequently occurring type of primary tumors of the central nervous system (CNS) mostly in adults, and its poor prognosis has not been significantly improved despite the fact that the innovative diagnostic strategies and new therapies have been developed. Glioblastoma is often located in a region of the forebrain known as the cerebrum, which controls some of the most advanced process such as speech and emotions. The exact underlying cause of Glioblastoma is unknown. Some cases may develop from existing, low-grade astrocytomas (malignant transformation) or they may occur without any evidence of a previous tumor (de novo).
Glioblastoma Diagnosis
The diagnosis of Glioblastoma includes neurological exams (this exam tests vision, hearing, speech, strength, sensation, balance, coordination, reflexes and the ability to think and remember), angiograms, magnetic resonance imaging (MRI) and computerized tomography (CT), surgical biopsy and others.
Glioblastoma Treatment
Glioblastoma treatment usually includes a combination of surgery, chemotherapy, radiation, stereotactic radiosurgery and Tumor treatment fields (TTF). Chemotherapy includes Carmustine (BCNU), Lomustine (CCNU), or Gleostine (Generic), Gliadel wafer (biodegradable discs infused with BCNU), Temozolomide (Temodar) Cisplatin, Carboplatin, Etoposide and Irinotecan. They may be given as a single agent or in combination i.e. PCV (Procarbazine, CCNU, and Vincristine), Carboplatin/ Etoposide. The drugs most commonly used to treat brain tumors are temozolomide (Temodar) and bevacizumab (Avastin).
Glioblastoma Multiforme Epidemiology
The disease epidemiology covered in the report provides historical as well as forecasted epidemiology segmented by Total Diagnosed Incident Population of Glioblastoma, Gender-specific Diagnosed Incidence of Glioblastoma, Type-specific Diagnosed Incidence of Glioblastoma, Age-specific Diagnosed Incidence of Glioblastoma, Diagnosed Incident Population based on Primary Site of Glioblastoma, and Diagnosed Incident Population based on Histologic Classification of Glioblastoma Tumor in the 7MM market covering the United States, EU-4 countries (Germany, France, Italy, and Spain), the United Kingdom and Japan from 2020 to 2034.
Key Findings
This section provides glimpse of the Glioblastoma epidemiology in the 7MM
Country Wise- Glioblastoma Multiforme Epidemiology
- The epidemiology segment also provides the Glioblastoma Prevalence data and findings across the United States, the EU-4 (Germany, France, Italy, and Spain), the United Kingdom and Japan.
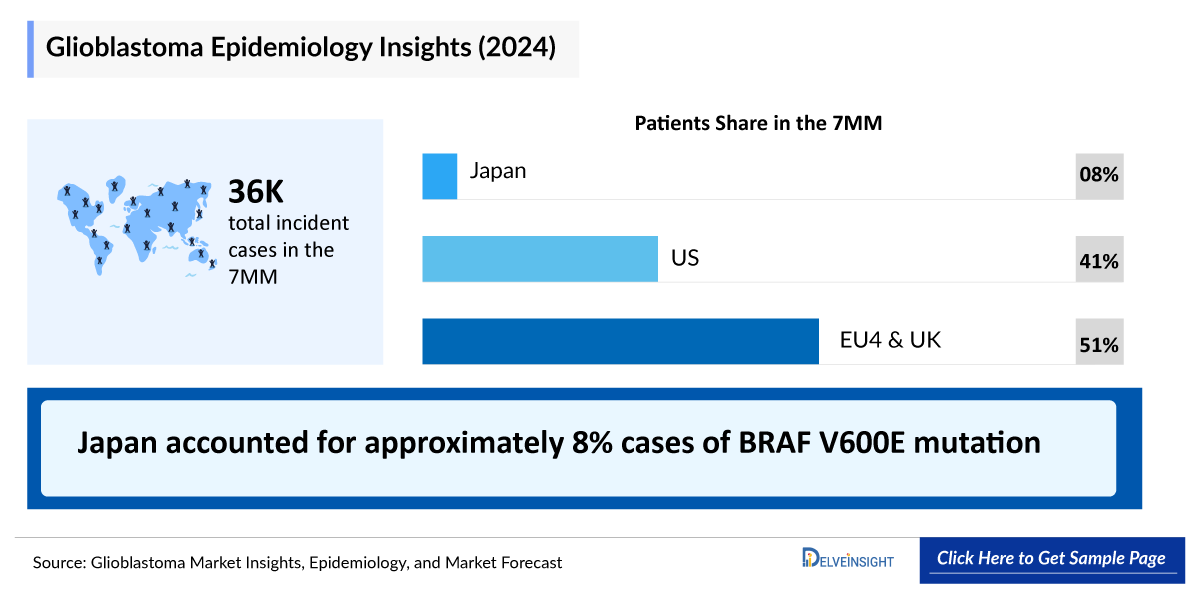
- The Total Glioblastoma Diagnosed Incident Population in the 7MM comprised of 32,546 cases in 2021 and are projected to increase during the forecast period.
- The Glioblastoma Gender-specific Diagnosed Incidence in the United States, in which maximum cases were recorded in male population i.e., 7,705 in 2021, as compared to female population.
- Among the EU-4, the UK countries, Germany accounted for the highest number of Glioblastoma cases, followed by France, whereas Spain accounted for the lowest cases in 2021.
- In Japan, the Glioblastoma Type-specific Diagnosed Incidence in Japan was of two types in which maximum cases were recorded of Primary Glioblastoma/ IDH-wild Type 2,541 in 2021 and is anticipated to rise during the forecast period.
Glioblastoma Recent Developments
- In September 2025, NeOnc Technologies Holdings, Inc. (NASDAQ: NTHI) announced that the FDA has authorized the company to advance to Phase IIa/IIb of its clinical trial for NEO212-01, a therapy targeting central nervous system (CNS) cancers.
- In Sept 2025, NeuroNOS, a Beyond Air subsidiary, received FDA Orphan Drug Designation for BA-101, its investigational therapy for glioblastoma (GBM). GBM is a highly aggressive brain tumor with poor prognosis, where current treatments like surgery, radiation, and temozolomide extend survival but are not curative.
- In July 2025, Mustang Bio announced FDA Orphan Drug Designation for MB-101, an IL13Ra2-targeted CAR T-cell therapy for recurrent astrocytomas and glioblastoma (GBM). The designation offers benefits like market exclusivity and trial incentives. Early Phase 1 results showed promising patient responses, and preclinical data support combining MB-101 with the oncolytic virus MB-108 for improved outcomes.
- In July 2025, Hemispherian AS announced that the FDA granted Orphan Drug Designation (ODD) to its drug candidate GLIX1 for the treatment of malignant glioma, a group of aggressive brain cancers that includes glioblastoma.
- In June 2025, Myosin Therapeutics announced FDA acceptance of its IND application for MT-125, enabling a Phase 1 trial evaluating the drug combined with radiation in patients with newly diagnosed IDH wild type, MGMT unmethylated glioblastoma, a group with poor prognosis and limited chemotherapy options.
- In May 2025, Shuttle Pharmaceuticals (Nasdaq: SHPH) announced it has nearly reached 50% enrollment in its Phase 2 trial of Ropidoxuridine for glioblastoma. The drug has been well tolerated with low toxicity (grade ≤2), and 84% of enrolled patients have completed all seven treatment cycles.
- In December 2024, Kazia Therapeutics was informed by the FDA that accelerated approval for its brain cancer drug, paxalisib, is unlikely. The FDA stated that the Phase II/III GBM-AGILE study's overall survival data, showing a 3.8-month improvement in glioblastoma patients, does not meet the criteria for accelerated approval.
- On October 15, 2024, the FDA granted Fast Track designation to LP-184, a small-molecule alkylating agent developed by Lantern Pharma for the treatment of glioblastoma (GBM). LP-184 induces tumor cell death through DNA damage and is currently in a Phase 1a trial assessing its safety and tolerability in patients with various solid tumors, including GBM. The Fast Track status aims to accelerate the development and review of LP-184, particularly given the limited treatment options for aggressive brain cancers like GBM.
Glioblastoma Drugs Market Chapters
Drug chapter segment of the Glioblastoma report encloses the detailed analysis of Glioblastoma marketed drugs and emerging (Phase-III and Phase II and Phase I/II) Glioblastoma pipeline drugs analysis. It also helps to understand the Glioblastoma clinical trials details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug and the latest news and press releases.
Glioblastoma Marketed Drugs
- Avastin: Genentech
Avastin (Bevacizumab) is a recombinant humanized monoclonal IgG1 antibody, which acts as angiogenesis inhibitor by blocking its target, vascular endothelial growth factor (VEGF). Bevacizumab binds to the vascular endothelial growth factor (VEGF) with its receptor VEGFR-1 and VEGFR-2, which are present on the surface of endothelial cells. In December 2017, the US FDA granted full approval of bevacizumab (Avastin) for the treatment of adults with recurrent Glioblastoma that has progressed following prior therapy.
- Temodar/Temodal: Merck
The active pharmaceutical ingredient in Temodar/Temodal, is an imidazotetrazine derivative of the alkylating agent dacarbazine. Temozolomide is used for the treatment of several brain cancer forms, e.g., as a second-line treatment for astrocytoma and as a first-line treatment for Glioblastoma. The therapeutic benefit of temozolomide is due to its ability to alkylate/methylate DNA. In July 2006, the Japan Ministry of Health, Labor and Welfare (MHLW) approved Temodal (R) (temozolomide) Capsules for the Malignant Glioma Treatment.
Note: Detailed Current therapies assessment will be provided in the full report of Glioblastoma
Glioblastoma Emerging Drugs
- Ofranergene obadenovec (VB-111): VBL Therapeutics
Ofranergene obadenovec (VB-111) is a first-in-class, targeted anticancer gene-therapy agent that is being developed by VBL Therapeutics to treat a wide range of solid tumors such as Glioblastoma. It is a non-replicating adenovirus 5 (Ad-5, El-deleted) carrying a proapoptotic human Fas-chimera transgene that targets angiogenic blood vessels and leads to vascular disruption. The drug has been rewarded Orphan Drug Designation from both US FDA and EMA for the treatment of patients with Glioblastoma. In addition, it has also been granted Fast Track Designation by the US FDA for prolongation of survival in patients with Glioblastoma. Currently, the drug has completed phase III stage of Glioblastoma clinical trials development for the patients with rGlioblastoma.
In November 2017, VBL Therapeutics signed an exclusive license agreement with NanoCarrier for the development, commercialization, and supply of ofranergene obadenovec (VB-111) in Japan.
- Trans Sodium Crocetinate: Diffusion Pharmaceuticals
Trans Sodium Crocetinate (TSC), being investigated by Diffusion Pharmaceuticals, is a first-in-class small molecule that, by its novel proprietary mechanism, safely reoxygenates oxygen-deprived tissue. It can act alone or with other treatments and presents opportunities in Glioblastoma unmet needs across several markets. It can be used to enhance the cancer-killing power of radiation and chemotherapy for treating patients with Glioblastoma.
- Selinexor (KPT-330): Karyopharm Therapeutics
Selinexor (KPT-330) is a first-in-class, oral Selective Inhibitor of Nuclear Export/SINE compound in development by Karyopharm Therapeutics. It functions by binding with and inhibiting the nuclear export protein XPO1 (also called CRM1), leading to the accumulation of tumor suppressor proteins in the cell nucleus, which subsequently reinitiates and amplifies their tumor suppressor function; this is supposed to lead to the selective induction of apoptosis in cancer cells, while largely sparing normal cells. The drug has been approved with a brand name Xpovio for patients in combination with dexamethasone for the treatment of adult patients with relapsed or refractory multiple myeloma (RRMM) who have received at least four prior therapies and whose disease is refractory to at least two proteasome inhibitors, at least two immunomodulatory agents, and an anti‐CD38 monoclonal antibody. The phase II (KING) study was terminated due to sponsor decision.
- VBI-1901: VBI Vaccines
VBI-1901 is a cancer vaccine that VBI Vaccines has designed to treat Glioblastoma and medulloblastoma, which are two types of brain tumors. It is designed to kill Glioblastoma and medulloblastoma tumor cells infected with cytomegalovirus or CMV. While the immune system already targets cells infected by viruses, VBI-1901’s goal is to boost their immune response such viruses. CMV inside tumor cells generate proteins called viral antigens that travel to the cells’ surface. Currently, the vaccine is in phase I/II stage of clinical development for the Recurrent Glioblastoma Patients and the company expects to initiate a randomized, controlled clinical study with registration potential in the fourth quarter of 2021. Recently, in June 2021, the company was granted Fast track designation by the FDA for VBI-1901 for the Recurrent Glioblastoma Treatment.
- ITI-1000 (pp65 DC Vaccine): Immunomic Therapeutics
Immunomic Therapeutics (ITI) is developing ITI-1000 (pp65 DC vaccine), which is a cancer cell vaccine consisting of autologous dendritic cells (DCs) loaded with mRNA encoding the human cytomegalovirus (CMV) matrix protein pp65 as a fusion protein with the short lysosome-associated membrane protein (shLAMP), with potential immunostimulatory and antineoplastic activities. ITI-1000 is currently being investigated in a phase II ATTAC-II trial for Glioblastoma Multiforme, Glioblastoma, Malignant Glioma, Astrocytoma, Grade IV, and Glioblastoma and is funded by the National Cancer Institute.
Glioblastoma Market Outlook
Glioblastoma treatment is quite challenging as some cells may respond well to certain therapies, while others may not be affected at all. Because of this, the treatment plan for glioblastoma may combine several approaches. The treatment often comprises a combination of several therapies, including surgery, chemotherapy, radiation, or stereotactic radiosurgery followed by the additional/adjuvant treatments, such as chemotherapy or radiation therapy, after surgery.
The first step in treating glioblastoma is a surgical procedure to make a diagnosis, to relieve pressure on the brain, and to remove as much tumor as possible safely. Glioblastoma surgery is performed to achieve a “maximum safe resection,” or removing as much of the tumor as possible without causing lasting neurological damage.
It is interesting to note that the emerging market of Glioblastoma includes budding gene therapy, i.e., Ofranergene obadenovec (VB-111) by VBL Therapeutics, followed by four vaccine/immunotherapy candidates such as VBI-1901, AV-Glioblastoma-1 and ITI-1000 (pp65 DC Vaccine), Tasadenoturev (DNX-2401) by VBI Vaccines, Aivita Biomedical, Immunomic Therapeutics, and DNAtrix, respectively.
The Glioblastoma pipeline possessed multiple potential drugs in late- and mid-stage developments to be launched shortly. Glioblastoma companies involved in robust research and development include Paxalisib (GDC-0084): Kazia Therapeutics, LAM561: Laminar Pharmaceuticals, Tasa-denoturev (DNX-2401): DNAtrix and, others are some of the major players that are going to alter the market dynamics in the coming years.
Key Findings
This section includes a glimpse of the Glioblastoma market size and share outlook in the 7MM.
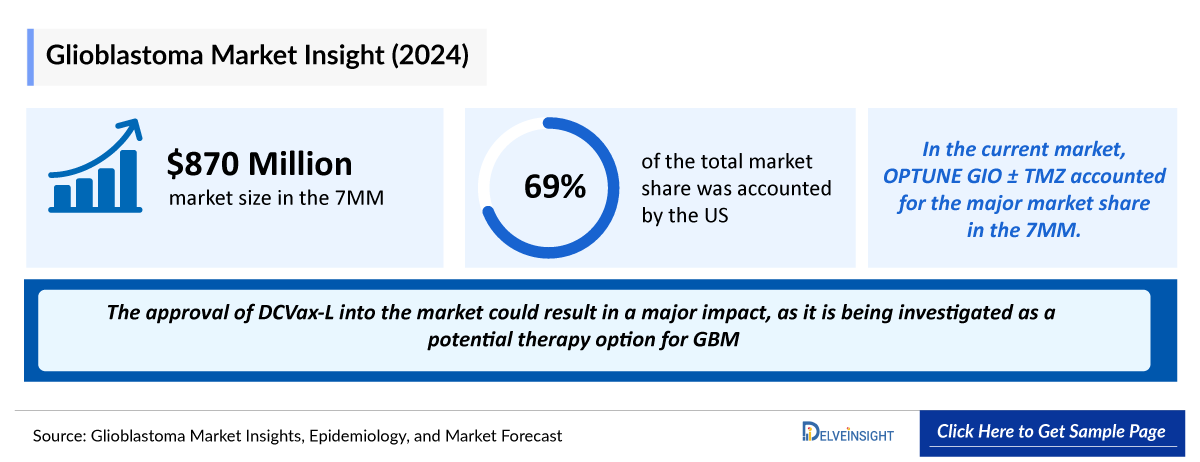
- The Glioblastoma Treatment Market Size in the 7MM is USD 799 million in 2021 and is projected to grow during the forecast period (2024-2034).
- According to the estimates, the highest Glioblastoma Treatment Market Size is from the United States, in 2021.
- Among the EU4 (Germany, France, Italy, Spain), and the UK, in which Germany has the maximum revenue share in 2021 while Spain has the lowest Glioblastoma market share.
- The Glioblastoma Treatment Market Size in Japan is USD 58 million in 2021 which is expected to rise during the forecast period (2024-2034).
The United States Glioblastoma Market Outlook
The total Glioblastoma Market Size in the United States is expected to increase with a CAGR of 13.1% during the study period (2020–2034).
EU-4 (Germany, France, Italy, and Spain), and the UK Market Outlook
The total Glioblastoma Market Size in EU-4, the UK is expected to increase with a CAGR of 13.5% during the study period (2020–2034).
Japan Glioblastoma Market Outlook
The total Glioblastoma Market Size in Japan is expected to increase with a CAGR of 10.4% during the study period (2020–2034).
Analyst Commentary
- The Glioblastoma Drugs Market is currently using Temozolomide (Temodar) and Bevacizumab (Avastin) as approved therapies, but the market lacks an effective strategy to cure glioblastoma, which provides a lucrative opportunity to develop more treatment options.
- Advancement in the understanding of molecular mechanisms and gene mutations are leading to more promising and tailored therapeutic approaches for Glioblastoma patients
- Owing to the launch of Bevacizumab biosimilars, a reduction in cost burden can be witnessed
- Despite a strong pipeline, most of the emerging therapies tend to fail in the high phase trials
- There is a substantial market opportunity for vaccines and oncolytic virus therapies in Glioblastoma
Glioblastoma Drugs Uptake
This section focusses on the rate of uptake of the potential Glioblastoma drugs expected to get launched in the market during the study period 2019-2032. The analysis covers Glioblastoma market uptake by drugs; patient uptake by therapies; and sales of each drug. For example- Kintara Therapeutics ‘VAL-083’ is a DNA-targeting agent, in three distinct biomarker-driven GMB patient populations. VAL-083 can cross the blood-brain barrier (BBB), has biological and tumor affecting activity against a range of cancers, including Glioblastoma and ovarian cancer. As per our analysis VAL-083, drug uptake in the US is expected to be medium-fast with peak share of 12%, years to peak would be 7 years in first line, whereas in second-line peak share is estimated to 5.5%.
Glioblastoma Pipeline Development Activities
The Glioblastoma therapeutics market report provides insights into different therapeutic candidates in Phase III, Phase II and Phase I/II stage. It also analyzes glioblastoma companies involved in developing targeted therapeutics.
Pipeline Development Activities
The Glioblastoma therapeutics market report covers the detailed information of collaborations, acquisition and merger, licensing and patent details for Glioblastoma emerging therapies.
KOL- Views
To keep up with current market trends, we take KOLs and SME's opinion working in the domain through primary research to fill the data gaps and validate our secondary research. Some of the leaders like Chief Executive Officer, VBL Therapeutics, Chief executive officer, DNAtrix, Chief Medical Officer, VBI Vaccines, Chief Medical Officer, Kazia Therapeutics and others. Their opinion helps to understand and validate current and emerging therapies treatment patterns or Glioblastoma market trend. This will support the clients in potential upcoming novel treatment by identifying the overall scenario of the market and the Glioblastoma unmet needs.
Competitive Intelligence Analysis
We perform competitive and market Intelligence analysis of the Glioblastoma therapeutics market by using various competitive intelligence tools that include–SWOT analysis, PESTLE analysis, Porter’s five forces, BCG Matrix, Market entry strategies, etc. The inclusion of the analysis entirely depends upon the data availability.
Glioblastoma Therapeutics Market Report Scope
- The Glioblastoma therapeutics market report covers the descriptive overview, explaining its causes, signs and symptoms, pathogenesis and currently available therapies.
- Comprehensive insight has been provided into the Glioblastoma epidemiology and treatment.
- Additionally, an all-inclusive account of both the current and emerging therapies for Glioblastoma are provided, along with the assessment of new therapies, which will have an impact on the current Glioblastoma treatment market landscape.
- A detailed review of Glioblastoma therapeutics market; historical and forecasted is included in the report, covering the 7MM drug outreach.
- The Patient-Based Glioblastoma Market Forecasting report provides an edge while developing business strategies, by understanding trends shaping and driving the 7MM Glioblastoma market.
Glioblastoma Treatment Market Report Highlights
- In the coming years, Glioblastoma treatment market is set to change due emerging therapies in the pipeline, and incremental healthcare spending across the world; which would expand the size of the market to enable the drug manufacturers to penetrate more into the market.
- The Glioblastoma companies and academics are working to assess challenges and seek opportunities that could influence Glioblastoma R&D. The therapies under development are focused on novel approaches to treat/improve the disease condition.
- As per DelveInsight’s analysis the subtypes- specific of Glioblastoma include type specific cases of Primary Glioblastoma/ IDH-wild Type and Secondary Glioblastoma/ IDH Mutant.
- Expected Launch of potential therapies, Paxalisib (GDC-0084): Kazia Therapeutics, LAM561: Laminar Pharmaceuticals, Tasa-denoturev (DNX-2401): DNAtrix and others might change the landscape in treatment of Glioblastoma.
Glioblastoma Treatment Market Report Insights
- Patient-Based Glioblastoma Market Forecasting
- Glioblastoma Therapeutic Approaches
- Glioblastoma Pipeline Drugs Analysis
- Glioblastoma Market Size and Trends
- Glioblastoma Treatment Market Opportunities
- Impact of upcoming Glioblastoma Therapies
Glioblastoma Treatment Market Report Key Strengths
- 11 Years Glioblastoma Market Forecast
- 7MM Coverage
- Glioblastoma Epidemiology Segmentation
- Key Cross Competition
- Highly Analyzed Glioblastoma Treatment Market
- Glioblastoma Drugs Uptake
Glioblastoma Treatment Market Report Assessment
- Current Glioblastoma Treatment Market Practices
- Glioblastoma Unmet Needs
- Glioblastoma Pipeline Drugs Analysis Profiles
- Glioblastoma Drugs Market Attractiveness
- Glioblastoma Market Drivers
- Glioblastoma Market Barriers
- SWOT
- Attribute Analysis
Key Questions Answered In the Glioblastoma Market Report
Glioblastoma Treatment Market Insights:
- What was the Glioblastoma drugs market share (%) distribution in 2020 and how it would look like in 2034?
- What would be the Glioblastoma treatment market size as well as market size by therapies across the 7MM during the study period (2020–2034)?
- What are the key findings pertaining to the market across the 7MM and which country will have the largest Glioblastoma market size during the study period (2020–2034)?
- At what CAGR, the Glioblastoma drugs market is expected to grow at the 7MM level during the study period (2020–2034)?
- What would be the Glioblastoma market outlook across the 7MM during the study period (2020–2034)?
- What would be the Glioblastoma market growth till 2034 and what will be the resultant market size in the year 2034?
- How would the market drivers, barriers and future opportunities affect the market dynamics and subsequent analysis of the associated trends?
Glioblastoma Epidemiology Insights:
- What is the disease risk, burden and Glioblastoma unmet needs?
- What is the historical Glioblastoma patient pool in the United States, the EU4 (Germany, France, Italy, and Spain), the UK and Japan?
- What would be the forecasted patient pool of Glioblastoma at the 7MM level?
- What will be the growth opportunities across the 7MM with respect to the patient population pertaining to Glioblastoma?
- Out of the above-mentioned countries, which country would have the highest incident population of Glioblastoma during the study period (2020–2034)?
- At what CAGR the population is expected to grow across the 7MM during the study period (2020–2034)?
Glioblastoma Current Treatment Scenario and Emerging Therapies:
- What are the current options for the treatment of Glioblastoma? What are the current treatment guidelines for the treatment of Glioblastoma in the US and Europe?
- How many companies are developing therapies for the treatment of Glioblastoma?
- How many emerging therapies are in the mid-stage and late stage of development for the treatment of Glioblastoma?
- What are the key collaborations (Industry–Industry, Industry–Academia), Mergers and acquisitions, licensing activities related to the Glioblastoma therapies?
- What are the recent novel therapies, targets, mechanisms of action and technologies developed to overcome the limitation of existing therapies?
- What are the clinical studies going on for Glioblastoma and their status?
- What are the key designations that have been granted for the emerging therapies for Glioblastoma?
- What are the 7MM historical and forecasted market of Glioblastoma?
Reasons to Buy
- The Patient-Based Glioblastoma Market Forecasting report will help in developing business strategies by understanding trends shaping and driving the Glioblastoma.
- To understand the future market competition in the Glioblastoma drugs market and Insightful review of the SWOT analysis of Glioblastoma.
- Organize sales and marketing efforts by identifying the best opportunities for Glioblastoma in the US, the EU-4 (Germany, Spain, Italy, and France) the United Kingdom and Japan.
- Identification of strong upcoming players in the Glioblastoma drugs market will help in devising strategies that will help in getting ahead of competitors.
- Organize sales and marketing efforts by identifying the best opportunities for Glioblastoma drugs market.
- To understand the future market competition in the Glioblastoma drugs market.
Stay Updated with Us for New Articles:-
- 13 of the most commonly asked questions about Glioblastoma multiforme, Answered
- Niacin fights Glioblastoma, iTeos nets $125M, Pandion reels in $80M, Brii, Chinese partner, FDA to speed up COVID-19 therapies
- DOC1021 – Unveiling Hope: Diakonos Oncology's Breakthrough in Glioblastoma Treatment: AACR 2024
- Safety and preliminary efficacy of AZD1390 + radiation therapy (RT) for glioblastoma (GBM)
- Glioblastoma Multiforme: Advancements in the Treatment Paradigm of the Malignant Condition
- Glioblastoma Multiforme Market: Emerging Pipeline Therapies To Keep A Keen Eye On
- Santhera flunks DMD phase 3; Scribe emerges with $20 M; Merck sells off Osteoarthritis drug to Novartis; Research updates on flu vaccine and glioblastoma
- Glioma vs. Glioblastoma Therapeutics Space: Unveiling the Battlefront
- Glioblastoma Multiforme Market: Infographics
- Latest DelveInsight Blogs