Glioblastoma Multiforme Market
- The Glioblastoma Multiforme Market Size in the 7MM was around USD 835 million in 2023 and is expected to increase with a significant CAGR during the forecast period.
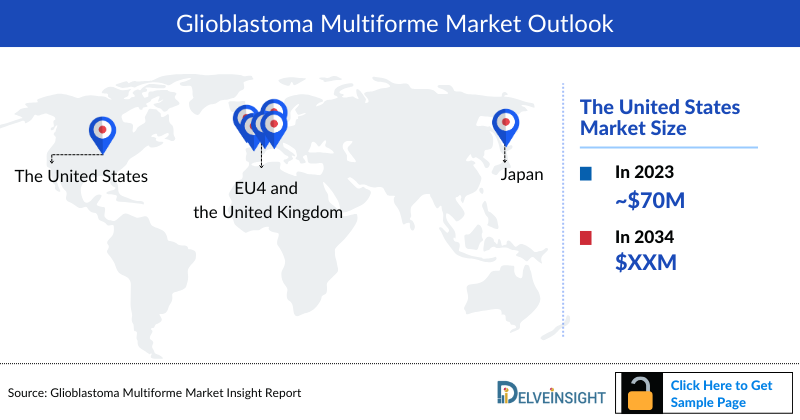
- Among the 7MM, the United States accounted for the largest Glioblastoma Multiforme Market Size, i.e., approximately 70% of the overall market in 2023.
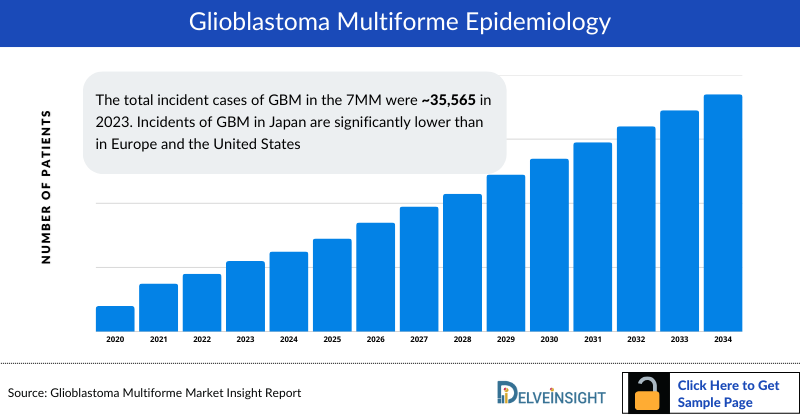
- The Glioblastoma Multiforme Incident Cases in the 7MM were ~35,565 in 2023. Glioblastoma Multiforme Incidents in Japan are significantly lower than in Europe and the United States
- Despite advancements in characterizing Glioblastoma Multiforme pathogenesis and potential therapeutic vulnerabilities, the standard of care for newly diagnosed Glioblastoma Multiforme of maximally safe surgery followed by radiation therapy with concurrent and adjuvant temozolomide chemotherapy has remained largely unchanged for decades.
- There have been very few therapies for Glioblastoma Multiforme approved by the United States Food and Drug Administration (FDA) over the past two decades because the clinical translation of novel findings on Glioblastoma Multiforme pathogenesis into drug discovery poses significant challenges. The brain is considered an “immunologically privileged site” because of the blood-brain barrier (BBB), and the Glioblastoma Multiforme tumor microenvironment causes further immunosuppression.
- While targeted therapies have been devised and clinically tested to tackle the molecular diversity of tumors, most have fallen short in improving survival rates.
- Upon recurrence, only about one in four patients can undergo repeat surgery due to concerns of morbidity, and other treatment options include repeat chemoradiation, anti-angiogenic agents (bevacizumab), tumor treating field therapy, and inclusion into clinical trials.
- The US FDA approved TAFINLAR + MEKINIST in June 2022 for the treatment of advanced tumors with a mutation called BRAF V600E. The approval includes use in both adult and pediatric (older than six years of age) high-and low-grade glioma patients with this mutation whose tumors progressed after prior treatment.
- Numerous cancer vaccines for 1L and 2L+ Glioblastoma Multiforme are in the development phases. Northwest Biotherapeutics, TVAX Biomedical, Aivita Biomedical, Inovio Pharmaceuticals, and many others are developing cancer vaccines for Glioblastoma Multiforme.
- OPTUNE GIO used along with temozolomide manufactured by Novocure is the market leader among augmentation therapies in Glioblastoma Multiforme based on 2023 sales.
Request for Unlocking the Sample Page of the "Glioblastoma Multiforme Treatment Market"
Key Factors Driving Glioblastoma Multiforme (GBM) Market
Glioblastoma Multiforme Patient Pool and Market Size
In 2023, the United States accounted for nearly 70% of the Glioblastoma Multiforme (GBM) market among the 7MM. Incident GBM cases were ~35.5K in 2023, with Japan reporting significantly lower numbers than Europe and the US.
Current Treatment Landscape
The standard of care for newly diagnosed GBM—surgery, radiotherapy, and temozolomide—has remained unchanged for decades. Treatment challenges include the blood–brain barrier and an immunosuppressive tumor microenvironment. Upon recurrence, only 25% of patients are eligible for repeat surgery, with other options including chemoradiation, bevacizumab, tumor treating fields (OPTUNE GIO), and clinical trials. FDA approvals are rare, though targeted options like TAFINLAR + MEKINIST (BRAF V600E mutations) have expanded the treatment scope.
Emerging Therapies and Pipeline Activity
The GBM pipeline is diverse, with inhibitors of PI3K, CDK4/6, VEGFR-2, PARP, IL4R, and NFkB under investigation. Immunotherapy is a major focus, with vaccines such as DCVax-L, VBI-1901, SurVaxM, and TVI-Brain-1 progressing in trials. The Glioblastoma AGILE platform trial is accelerating development for companies including Kazia, Kintara, Biohaven, and Vigeo. While regorafenib and VAL-083 were discontinued, paxalisib showed a survival benefit in unmethylated GBM, and Vigeo’s VT1021 is advancing into Phase III.
DelveInsight’s "Glioblastoma Multiforme Market Insight, Epidemiology, and Market Forecast – 2034" report delivers an in-depth understanding of Glioblastoma Multiforme, historical and forecasted epidemiology as well as the Glioblastoma Multiforme market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
The Glioblastoma Multiforme Treatment Market Report provides current treatment practices, emerging drugs, Glioblastoma Multiforme market share of individual therapies, and current and forecasted Glioblastoma Multiforme market size from 2020 to 2034, segmented by seven major markets. The report also covers current Glioblastoma Multiforme treatment market practices/algorithms and unmet medical needs to curate the best of the opportunities and assess the underlying potential of the market.
Glioblastoma Multiforme Treatment Market: Understanding and Algorithm
Glioblastoma Multiforme is the most frequently occurring type of primary tumor of the central nervous system (CNS) mostly in adults, and its poor prognosis has not been significantly improved although innovative diagnostic strategies and new therapies have been developed. Somatic evolution promotes the progression of cancer in which the genome of the cancer cell is being deviated from that of the healthy cell due to the accumulation of mutations. There is a remarkable development in Glioblastoma Multiforme because it occurs via a complex network of different molecular and genetic aberrations, which leads to significant changes in major signaling pathways. Glioblastoma Multiformes, as they extensively disperse throughout the parenchyma, making maximal surgical resection unattainable and having a high level of vascularization, are lethal.
Glioma is considered the general term that is used to describe primary brain tumors, and it is also classified according to their presumed cell of origin accordingly.
Glioblastoma Multiforme Diagnosis
The clinical presentation of glioblastoma varies based on factors such as tumor size, location, and the extent of peritumoral edema. The primary diagnostic tool for glioblastoma is contrast-enhanced magnetic resonance imaging (MRI), which is the most commonly used non-invasive technique. For more precise imaging, positron emission tomography (PET) is often recommended, particularly for diagnosing grade III/IV glioblastoma. An innovative approach gaining traction is immunotargeted imaging, which involves using highly specific antibodies that bind to tumor cell surface targets, followed by PET imaging to visualize the tumor. This technique enables real-time monitoring, offering a promising advancement in glioblastoma diagnosis and management.
Further details related to diagnosis will be provided in the report…
Glioblastoma Multiforme Treatment
Glioblastoma Multiforme treatment usually includes a combination of surgery, chemotherapy, radiation, or stereotactic radiosurgery. Surgery is usually one of the most important aspects of treatment, although rarely used alone. Since glioblastomas develop very rapidly, they are often difficult to remove in their entirety. Therefore, surgery is performed to achieve a maximum safe resection – removing as much of the tumor as possible while preserving the patient’s brain function and sparing healthy tissues. After surgery, residual cancer cells can be targeted with additional treatments, such as chemotherapy or radiation therapy. Radiation therapy and chemotherapy usually follow surgery once the diagnosis or name of the tumor is determined. Because this multispecialty approach can cause several side effects, steroids are often provided as another essential part of glioblastoma treatment, used to help alleviate the side effects of other therapies. Steroid treatment can be used to reduce swelling or antiseizure medication.
Glioblastoma Multiforme Epidemiology
The Glioblastoma Multiforme epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by the Total Incident Population of Glioblastoma Multiforme, Gender-specific Incidence Cases of Glioblastoma Multiforme, Type-specific Incidence Cases of Glioblastoma Multiforme, Incident Cases based on Primary Site of Glioblastoma Multiforme, Age-specific Incidence Cases of Glioblastoma Multiforme, Incident Cases Based on Histologic Classification of Glioblastoma Multiforme Tumor, Unmethylation of the MGMT Gene Promoter Cases, BRAF V600E Mutation Cases, and Line-wise Treated Pool of Glioblastoma Multiforme in the 7MM covering the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan from 2020 to 2034.
- Among the 7MM, the US accounted for approximately 41%, EU4 and the UK for 51%, and Japan for 8% of the total Glioblastoma Multiforme Incident Cases in 2023.
- In the EU4 and the UK, in 2023, the maximum number of Glioblastoma Multiforme Incident Cases according to the histological classification was for glioblastoma with approximately 17,550 cases while the lowest incident cases were of giant cell glioblastoma type with approximately 150 cases, which are expected to increase by 2034.
- In Japan, in 2023, the highest number of cases in the targeted therapy pool was for first-line treatments, with 2,690 cases, followed by second-line and above treatments with 1,365 cases.
- As per the DelveInsight estimates, it has been found that the primary site of Glioblastoma Multiforme included maximum cases at the parietal site, while the minimum number of cases were found in unknown and other sites. This trend is evident across all the 7MM countries for the study period.
Glioblastoma Multiforme Marketed Drugs
- AVASTIN (bevacizumab): Roche (Genentech)
AVASTIN is a recombinant humanized monoclonal IgG1 antibody, which acts as an angiogenesis inhibitor by blocking its target, vascular endothelial growth factor (VEGF). It binds to the VEGF with its receptors VEGFR-1 and VEGFR-2, which are present on the surface of endothelial cells. This helps reduce VEGF activity and regress the vascularization of tumors, normalizing the tumor vasculature and inhibiting the formation of new tumor vasculature, thereby preventing tumor growth. VEGF is a chemical signal that stimulates angiogenesis in various diseases, especially cancer.
AVASTIN is indicated for treating Glioblastoma Multiforme with progressive disease in adult patients following prior therapy.
- In May 2009, the US FDA granted accelerated approval to AVASTIN injection as a single agent for patients with Glioblastoma Multiforme with progressive disease following prior therapy.
- In June 2013, Roche announced that the Japanese Ministry of Health, Labour and Welfare (MHLW) approved AVASTIN for the treatment of malignant glioma, including newly diagnosed Glioblastoma Multiforme in combination with radiotherapy and temozolomide chemotherapy, and as monotherapy for the treatment of recurrent Glioblastoma Multiforme (rGlioblastoma Multiforme) and certain other types of high-grade glioma following prior therapy in Japan.
- TEMODAR/TEMODAL (temozolomide): Merck
The active pharmaceutical ingredient in TEMODAR/TEMODAL is an imidazotetrazine derivative of the alkylating agent dacarbazine. It is used for treating several brain cancer forms, e.g., as a second-line treatment for astrocytoma and a first-line treatment for Glioblastoma Multiforme. The therapeutic benefit of TEMODAR is its ability to alkylate/methylate DNA. This alkylation/methylation destroys the DNA and triggers the death of the tumor cells. TEMODAR targets tumoral tissues selectively; it has an anti-neoplastic effect; it has minimum influence on adjacent brain tissues; it has no severe systemic toxicity; and it is eliminated rapidly.
TEMODAR was granted the first US FDA approval in the treatment of recurrent anaplastic astrocytoma in 1999, with subsequent approval for the first-line therapy of Glioblastoma Multiforme. In March 2005, the US FDA approved TEMODAR for the treatment of adult patients with newly diagnosed Glioblastoma Multiforme concomitantly with radiotherapy and then as maintenance treatment.
- In June 2005, TEMODAL received marketing approval in the EU for the treatment of patients with newly diagnosed Glioblastoma Multiforme concomitantly with radiotherapy and subsequently as monotherapy treatment.
- In July 2006, the Japan Ministry of Health, Labor and Welfare (MHLW) approved TEMODAL capsules for the treatment of malignant glioma. In January 2010, the MHLW approved TEMODAL Injection for the treatment of malignant glioma.
Glioblastoma Multiforme Emerging Drugs
- DCVax-L: Northwest Biotherapeutics and Advent BioServices
DCVax-L is a fully personalized immune therapy made from a patient’s immune cells (dendritic cells) and antigens (biomarkers) from a sample of the patient’s tumor. DCVax-L is expected to be used for any solid tumor cancers in situations where the patient has their tumor surgically removed as part of standard care. DCVax -L is administered to the patient through a simple intradermal injection in the upper arm, similar to a flu shot. The dendritic cells then convey the tumor biomarker information to the rest of the immune system agents (T cells, B cells, and others) as “marching orders,” and the immune system agents fan out through the body, searching for anything with these biomarkers and attacking it.
In August 2022, the company received approval from the UK MHRA for the Company’s Pediatric Investigation Plan (PIP). The development, regulatory review, and regulatory approval of a PIP is a prerequisite for the application for approval of new medicine for adult patients. The company’s approved PIP includes two clinical trials: one for newly diagnosed pediatric high-grade glioma and one for recurrent pediatric high-grade glioma.
- TVI-Brain-1: TVAX Biomedical
TVI-Brain-1 is a patented vaccine-enhanced adoptive T-cell therapy (VACT) of TVAX Biomedical containing attenuated autologous cancer cells and activated autologous blood-derived T cells, developed through TVAX’s immunotherapeutic cancer treatment platform. TVAX Immunotherapy is a proprietary method for treating cancer using many activated, genetically unique cancer-specific killer T cells. This vaccination generates an immune response in the patient, producing many cancer-specific T cells. The activated killer T cells trigger the body’s immune system to destroy cancer cells, including cancer stem cells.
- In June 2024, Capital Health Cancer Center announced that it had joined the TVAX Biomedical clinical trial to study a potential novel therapy for Glioblastoma Multiforme, the most common type of malignant brain cancer. Capital Health Cancer Center is one of five clinical sites open in the United States and currently, the only East Coast location north of Florida to offer access to the TVAX trial.
- In September 2022, TVAX Biomedical received an approximately USD 2 million 4-year grant from the FDA Office of Orphan Products for its planned glioblastoma study.
Glioblastoma Multiforme Drugs Market Insights
Few targeted therapies inhibit specific molecular targets involved in signaling pathways. A few common targets include EGFR (epidermal growth factor receptor), mTOR (mammalian target of rapamycin), PI3K (phosphatidylinositol 3-kinase), and VEGF (vascular endothelial growth factor). AVASTIN belongs to VEGF inhibitors. Numerous clinical trials are testing new therapeutic approaches with tyrosine kinase inhibitors and angiogenesis inhibitors.
Vaccines
The emerging landscape of Glioblastoma Multiforme treatment is witnessing a surge in immunotherapy and vaccine candidates such as DCVax-L, AV-Glioblastoma Multiforme-1, SurVaxM, TVI-Brain-1, and VBI-1901. Incorporating DC vaccination into the first-line combined treatment for Glioblastoma Multiforme is challenging. Most researchers have initiated DC vaccination shortly after radiochemotherapy, during the maintenance chemotherapy phase. However, the immunization effects of chemotherapy appear to be unequivocal. DC vaccination after TMZm instead of during TMZm resulted in a slightly better 2-year OS. Out of all these vaccines in the pipeline, SurVaxM showed superior efficacy. At the same time, DCVax-L stands out among emerging vaccines as the only one being investigated for both newly diagnosed and recurrent Glioblastoma Multiforme, showing significant survival benefits in both lines.
Further detailed analysis will be provided in the report….
Glioblastoma Multiforme Market Outlook
Glioblastoma is a malignant brain tumor that develops from a specific type of brain cell called an astrocyte. These cells nourish neurons (nerve cells of the brain) and form scar tissue that helps repair brain damage in response to injury. Glioblastomas are often very aggressive and grow into surrounding brain tissue. Unfortunately, there is no cure for glioblastoma. Glioblastoma treatment is quite challenging as some cells may respond well to certain therapies while others may not be affected at all. Because of this, the treatment plan for glioblastoma may combine several approaches.
The treatment often comprises a combination of several therapies, including surgery, chemotherapy, radiation, or stereotactic radiosurgery, followed by additional/adjuvant treatments, such as chemotherapy or radiation therapy, after surgery. The Glioblastoma Multiforme pipeline is robust and possesses multiple potential drugs in late and mid-stage developments, which are yet to be launched. The pipeline involves drugs with varied mechanisms of action along with different routes of administration, ranging from oral, IV, intratumoral, SC, etc.
It is interesting to note that the emerging Glioblastoma Multiforme therapeutics market includes vaccine/immunotherapy candidates such as DCVax-L, VBI-1901, AV-Glioblastoma Multiforme-1, SurVaxM, and TVI-Brain-1, VBI-1901, AV-Glioblastoma Multiforme-1, and SurVaxM respectively. Several companies are investigating their products under the Glioblastoma Adaptive Global Innovative Learning Environment (Glioblastoma Multiforme AGILE) Phase II/III platform, including Kazia Therapeutics, Kintara Therapeutics, Biohaven Pharmaceuticals, Vigeo Therapeutics, and Polaris Pharmaceuticals. Bayer's regorafenib and VBL Therapeutics' VAL-083 did not meet their primary endpoints, leading both companies to discontinue their products from the Glioblastoma Multiforme AGILE platform.
Similarly, Kazia Therapeutics reported Phase II/III Glioblastoma Multiforme-AGILE trial results for paxalisib, showing clinically meaningful overall survival improvements in newly diagnosed unmethylated glioblastoma patients, with full data expected later this year. No efficacy signal was observed in recurrent Glioblastoma Multiforme (median OS: 9.69 months SOC vs. 8.05 months paxalisib). Further analysis is underway. Meanwhile, Vigeo Therapeutics is currently enrolling patients as an arm of AGILE, a Phase III registration-ready clinical trial in Glioblastoma for VT1021.
- Among the 7MM, the US accounted for the largest Glioblastoma Multiforme Treatment Market Size. i.e., USD ~70 million in 2023.
- Among EU4 and the UK, Germany accounted for the highest Glioblastoma Multiforme Market Size in 2023, while Spain occupied the lowest.
- The Glioblastoma Multiforme Pipeline consists of an ornithine decarboxylase inhibitor, activator of sphingomyelin synthase I (SMSI) and inactivating key Ras-dependent proliferation pathways, PI3K pathway inhibitor, Interleukin-IV receptor (IL4R), CDK4/6 inhibitor, NFkB and HIF-Ia inhibitor, DNA inhibitors, VEGFR-2 inhibitor, PARP inhibitor, and others.
- In 2034, among all the emerging therapies, the highest revenue is expected to be generated by DCVax-L in the 7MM.
Further details will be provided in the report….
Recent Developments in the Glioblastoma Multiforme Treatment Market
- In November 2024, Kazia Therapeutics announced that the FDA has granted a Type C meeting scheduled for December 2024 to discuss potential pathways for the registration of the company’s blood-brain barrier-penetrant PI3K/mTOR inhibitor, paxalisib, for the treatment of patients with newly diagnosed Glioblastoma Multiforme.
- In April 2024, Enterome announced the successful completion of the Phase II ROSALIE study of EO2401 in recurrent glioblastoma.
- In November 2024, Novocure announced that the US FDA approved its new HFE transducer arrays for use with OPTUNE GIO for the treatment of adult patients with Glioblastoma Multiforme.
- In March 2024, the US FDA cleared TME Pharma’s IND application for NOX-A12 based on the protocol for its upcoming randomized Phase II trial in Glioblastoma Multiforme.
- CNS Pharmaceuticals announced that the enrollment of the Phase II trial of berubicin was completed in a potentially pivotal Glioblastoma Multiforme study evaluating berubicin, and the topline data is expected in the first half of 2025.
To be continued in the report….
Glioblastoma Multiforme Drugs Uptake
This section focuses on the uptake rate of potential Glioblastoma Multiforme drugs expected to be launched in the market during 2020–2034. The Glioblastoma Multiforme treatment market landscape has experienced a profound transformation with the uptake of novel drugs. These innovative therapies are redefining standards of care. Furthermore, the increased uptake of these transformative drugs is a testament to the unwavering dedication of physicians, oncology professionals, and the entire healthcare community in their tireless pursuit of advancing cancer care. This momentous shift in treatment paradigms is a testament to the power of research, collaboration, and human resilience.
Further detailed analysis of emerging therapies drug uptake in the report…
Glioblastoma Multiforme Pipeline Development Activities
The Glioblastoma Multiforme therapeutics market report provides insights into different therapeutic candidates in Phase III, Phase II/III, Phase II, PhaseI/II, and Phase I. It also analyzes key Glioblastoma Multiforme Companies involved in developing targeted therapeutics.
Pipeline Development Activities
The Glioblastoma Multiforme therapeutics market report covers detailed information on collaborations, acquisitions and mergers, licensing, and patent details for Glioblastoma Multiforme emerging therapies.
KOL- Views
To keep up with current market trends, we take KOLs and SMEs' opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Some of the leaders like MD, PhD, Research Project Manager, Director, and others. Their opinion helps to understand and validate current and emerging therapies and treatment patterns or Glioblastoma Multiforme market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the Glioblastoma Multiforme unmet needs.
Delveinsight’s analysts connected with 20+ KOLs to gather insights; however, interviews were conducted with 10+ KOLs in the 7MM. Centers such as the RWTH Aachen University Hospital, University of Valencia, Vall d'Hebron University Hospital, Drexel University, Saint Louis University, University of Birmingham, Juntendo University, Kyoto University, etc., were contacted. Their opinion helps understand and validate Glioblastoma Multiforme epidemiology and market trends.
Glioblastoma Multiforme Drugs Market: Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT and conjoint analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving Glioblastoma Multiforme treatment market landscape.
The analyst analyzes multiple emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. In efficacy, the trial’s primary and secondary outcome measures are evaluated. Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials.
Glioblastoma Multiforme Therapeutics Market Access and Reimbursement
AVASTIN
With the Genentech Oncology Co-pay Assistance Program, eligible patients with commercial insurance could pay as little as USD 0 per treatment for AVASTIN. Co-pay assistance of up to USD 25,000 is provided per calendar year.
Patients are eligible if:
- Taking AVASTIN for an FDA-approved use.
- They are 18 years of age or older or have a Legally Authorized Person over the age of 18 to manage the program.
- Have commercial (private or non-governmental) insurance. This includes plans available through state and federal health insurance exchanges.
- Live and receive treatment in the United States or US Territories.
- Are not receiving assistance through the Genentech Patient Foundation or any other charitable organization for the same expenses covered by the program.
- Do not use a state or federal healthcare plan to pay for medication. This includes, but is not limited to, Medicare, Medicaid, and TRICARE.
Independent Co-pay Assistance Foundations
An independent co-pay assistance foundation is a charitable organization providing financial assistance to patients with specific disease states, regardless of treatment. Patients who are commercially or publicly insured, including those covered by Medicare and Medicaid, can contact the foundations directly to request assistance. Eligibility requirements, all aspects of the application process, turnaround times, and the type or amount of assistance available (if any) can vary by foundation.
- CancerCare Co-Payment Assistance Foundation
- Good Days from CDF
- Patient Access Network Foundation (PANF)
- Patient Advocate Foundation (PAF)
- The Assistance Fund
- The HealthWell Foundation
- Independent co-pay assistance foundations have their own eligibility rules. They have no involvement or influence in independent foundation decision-making or eligibility criteria and do not know if a foundation will be able to help. They can only refer the patients to a foundation that supports the disease state.
Genentech Patient Foundation
The Genentech Patient Foundation gives free AVASTIN to people who have been prescribed this medicine and do not have insurance or who have financial concerns and meet specific eligibility criteria.
The patients are eligible if their insurance coverage and income match one of these situations:
- Uninsured patients with incomes under USD 150,000.
- Insured patients without coverage for AVASTIN with incomes under USD 150,000.
Insured patients with coverage for a Genentech medicine:
- With an out-of-pocket maximum set by their health insurance plan that exceeds 7.5% of their household income.
With household size and income within certain guidelines
- For any of these situations, add USD 25,000 for each extra person in households larger than four people.
Further detailed analysis will be provided in the report…
Glioblastoma Multiforme Therapeutics Market Report Scope
- The Glioblastoma Multiforme therapeutics market report covers a descriptive overview, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight has been provided into Glioblastoma Multiforme epidemiology and treatment.
- Additionally, an all-inclusive account of both the current and emerging therapies for Glioblastoma Multiforme is provided, along with the assessment of new therapies, which will have an impact on the current Glioblastoma Multiforme treatment market landscape.
- A detailed review of the Glioblastoma Multiforme treatment market; historical and forecasted is included in the report, covering the 7MM drug outreach.
- The Glioblastoma Multiforme therapeutics market report provides an edge while developing business strategies, by understanding trends shaping and driving the 7MM Glioblastoma Multiforme market.
Glioblastoma Multiforme Therapeutics Market Report Insights
- Patient-based Glioblastoma Multiforme Market Forecasting
- Therapeutic Approaches
- Glioblastoma Multiforme Pipeline Drugs Analysis
- Glioblastoma Multiforme Market Size and Trends
- Glioblastoma Multiforme Drugs Market Opportunities
- Impact of Upcoming Therapies
Glioblastoma Multiforme Therapeutics Market Report Key Strengths
- 11 Years Glioblastoma Multiforme Market Forecast
- 7MM Coverage
- Glioblastoma Multiforme Epidemiology Segmentation
- Key Cross Competition
- Highly Analyzed Glioblastoma Multiforme Drugs Market
- Glioblastoma Multiforme Drugs Uptake
Glioblastoma Multiforme Therapeutics Market Report Assessment
- Current Glioblastoma Multiforme Treatment Market Practices
- Glioblastoma Multiforme Unmet Needs
- Glioblastoma Multiforme Pipeline Drugs Analysis Profiles
- Glioblastoma Multiforme Drugs Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
FAQs
- What was the Glioblastoma Multiforme drugs market share (%) distribution in 2020 and what it would look like in 2034?
- What would be the Glioblastoma Multiforme treatment market size as well as market size by therapies across the 7MM during the study period (2020–2034)?
- Which country will have the largest Glioblastoma Multiforme market size during the study period (2020–2034)?
- What are the disease risks, burdens, and Glioblastoma Multiforme unmet needs?
- What is the historical Glioblastoma Multiforme patient pool in the United States, EU4 (Germany, France, Italy, and Spain), and the UK, and Japan?
- What will be the growth opportunities across the 7MM concerning the patient population of Glioblastoma Multiforme?
- How many emerging therapies are in the mid-stage and late stage of development for the treatment of Glioblastoma Multiforme?
- What are the key collaborations (Industry–Industry, Industry-Academia), Mergers and acquisitions, and licensing activities related to Glioblastoma Multiforme therapies?
- What are the recent novel therapies, targets, mechanisms of action, and technologies developed to overcome the limitations of existing therapies?
- What are the clinical studies going on for Glioblastoma Multiforme and their status?
- What are the key designations that have been granted for the emerging therapies for Glioblastoma Multiforme?
Reasons to Buy
- The Glioblastoma Multiforme therapeutics market report will help in developing business strategies by understanding trends shaping and driving Glioblastoma Multiforme.
- To understand the future market competition in the Glioblastoma Multiforme drugs market and Insightful review of the SWOT analysis of Glioblastoma Multiforme.
- Organize sales and marketing efforts by identifying the best opportunities for Glioblastoma Multiforme in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identification of strong upcoming players in the Glioblastoma Multiforme drugs market will help in devising strategies that will help in getting ahead of competitors.
- Organize sales and marketing efforts by identifying the best opportunities for the Glioblastoma Multiforme drugs market.
- To understand the future market competition in the Glioblastoma Multiforme drugs market.
Stay Updated with us for Recent Articles:-
- 13 of the most commonly asked questions about Glioblastoma multiforme, Answered
- Glioblastoma Multiforme: Advancements in the Treatment Paradigm of the Malignant Condition
- Glioblastoma Multiforme Market: Emerging Pipeline Therapies To Keep A Keen Eye On
- Huge Unmet Needs in the Glioblastoma Multiforme Treatment Market Driving the Market Size Growth
- Glioma vs. Glioblastoma Therapeutics Space: Unveiling the Battlefront
- Glioblastoma Multiforme Market: Infographics


-pipeline.png&w=256&q=75)




