Hodgkins Lymphoma Market
Key Highlights
- The total market size in the 7MM for Hodgkin lymphoma is expected to grow with a significant CAGR during the forecast period (2026-2036).
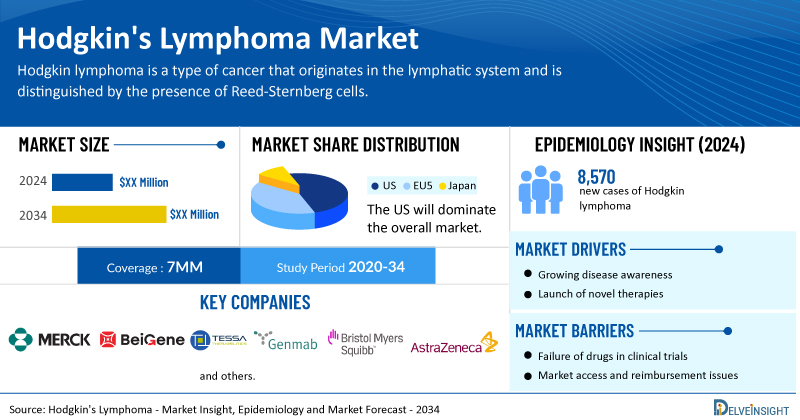
- In 2023, the US accounted for the maximum share of the total market in the 7MM.
- In 2024, an estimated 8,570 (4,630 in males and 3,940 in females) new cases of Hodgkin lymphoma are expected in the United States.
- Hodgkin lymphoma generally has a positive outlook, with majority of patients achieving long-term remission under current treatment protocols.
- The integration of chemotherapy, radiation therapy, and emerging treatments enables personalized treatment strategies tailored to the unique needs of each patient, optimizing therapeutic efficacy and improving overall care.
- In the UK Hodgkin lymphoma incidence rates increase significantly during childhood, peaking between ages 20 and 29. After this peak, the rates fluctuate, with notable rises at ages 35-39 and again between 75 and 84.
- The highest incidence rates are seen in females aged 20-24 and males aged 75-79. Over 80% of Hodgkin lymphoma patients can be cured with current treatment approaches. The cure rate is even higher, nearing 90%, for younger patients and those with early-stage favorable disease.
- In October 2023, European Commission Approves ADCETRIS (brentuximab vedotin) for the treatment of adult patients with previously untreated CD30+ Stage III Hodgkin Lymphoma in combination with doxorubicin, vinblastine and dacarbazine (AVD).
- The Hodgkin lymphoma has a strong pipeline, with many companies actively developing therapies. Key players include Merck, Bristol Myers Squibb, Takeda, Pfizer, Genmab and others.
DelveInsight’s “Hodgkin Lymphoma – Market Insights, Epidemiology, and Market Forecast – 2036” report delivers an in-depth understanding of Hodgkin lymphoma, historical and forecasted epidemiology as well as Hodgkin lymphoma market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
The Hodgkin lymphoma market report provides current treatment practices, emerging drugs, market share of individual therapies, and current and forecasted 7MM Hodgkin Lymphoma market size from 2022 to 2036. The report also covers current Hodgkin lymphoma treatment practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s potential.
Geography Covered
- The United States
- EU4 (Germany, France, Italy, and Spain) and the United Kingdom
- Japan
|
Study Period |
2022–2036 |
|
Forecast Period |
2026–2036 |
|
Geographies Covered |
US, EU4 (Germany, France, Italy, and Spain) and the UK, and Japan |
|
Hodgkin Lymphoma Epidemiology |
Segmented by:
|
|
Hodgkin Lymphoma Key Companies |
|
|
Hodgkin Lymphoma Key Therapies |
|
|
Hodgkin Lymphoma Market |
Segmented by:
|
|
Analysis |
|
Hodgkin Lymphoma: Understanding and Treatment Algorithm
Hodgkin Lymphoma Overview
Hodgkin lymphoma is a cancer that starts in the lymphatic system, defined by the presence of Reed-Sternberg cells. It is one of two main types of lymphoma, the other being non-Hodgkin lymphoma, and is known for its high cure rates when detected and treated early. In Hodgkin lymphoma, B-lymphocytes multiply abnormally and accumulate in areas like the lymph nodes, losing their ability to fight infections, which increases vulnerability to illness. The two main types are classic Hodgkin lymphoma and nodular lymphocyte-predominant Hodgkin lymphoma. While Hodgkin lymphoma is aggressive and can spread quickly, it is also one of the most treatable cancers.
Hodgkin Lymphoma Diagnosis
The initial diagnosis of Hodgkin lymphoma involves several techniques, including a biopsy of affected tissue or lymph nodes. Immunophenotyping is performed on the tissue to analyze the proteins expressed by the cells. Imaging tests such as CT scans, X-rays, and PET scans of the chest, abdomen, and pelvis are used to identify any enlargement of the spleen or lymph nodes, or to detect abnormal retinal veins. PET scans help identify patients who may only need chemotherapy, sparing them from the potential risks of radiotherapy, such as cardiac disease or secondary cancers. Blood tests are also conducted to assess overall health, evaluate the levels of red and white blood cells, and check platelet counts, as well as to monitor the function of organs like the liver and kidneys. Additionally, Fluorescence in Situ Hybridization (FISH) is used to examine genes or chromosomes in cells and tissues, helping to detect specific chromosomal abnormalities.
Following testing, the next step is to determine if the cancer has spread within the lymphatic system or to other parts of the body. Staging refers to assessing the extent of the cancer's spread. Hodgkin lymphoma is staged from stage I, where only one lymph node region or structure is involved, to stage IV, where the cancer has spread beyond the lymphatic system.
Further details related to diagnosis are provided in the report…
Hodgkin Lymphoma Treatment
Current treatment for Hodgkin lymphoma involves a combination of chemotherapy, radiation therapy, and targeted therapies, tailored to the disease's stage and subtype. In advanced-stage disease, chemotherapy regimens like ABVD (Adriamycin, Bleomycin, Vinblastine, and Dacarbazine) are commonly used. Targeted therapies such as ADCETRIS, and immunotherapies like OPDIVO and KEYTRUDA are also an integral to treatment, particularly for relapsed or refractory cases. Despite recent advancements, the Hodgkin lymphoma pipeline faces challenges with a relatively slow pipeline of new therapies. Limited novel drug candidates in development could hinder future treatment innovation and improvements in patient outcomes.
Further details related to treatment are provided in the report…
Hodgkin Lymphoma Epidemiology
The Hodgkin lymphoma epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by total Incident Cases of Hodgkin lymphoma, stage-specific incident cases of Hodgkin lymphoma, type-specific incident cases of Hodgkin lymphoma, age-specific incident cases of Hodgkin lymphoma, gender-specific incident cases of Hodgkin lymphoma, and treatable cases of Hodgkin lymphoma in the 7MM covering the United States, EU4 (Germany, France, Italy, and Spain), United Kingdom, and Japan from 2022 to 2036.
- The total number of incident cases of Hodgkin lymphoma in the 7MM was maximum in the US during the forecasted period.
- Hodgkin lymphoma is rare in children younger than 5 years old. But it's the most common cancer diagnosed in adolescent’s ages 15 to 19 years.
- In the UK, 42% of Hodgkin lymphoma cases occur in females, while 58% occur in males.
- The distribution of classical Hodgkin lymphoma subtypes is as follows: nodular sclerosis classical Hodgkin lymphoma (70%), mixed cellularity classical HL (25%), lymphocyte-rich classical Hodgkin lymphoma (5%), and lymphocyte-depleted classical HL (less than 1%). Nodular lymphocyte-predominant Hodgkin lymphoma (NLPHL) accounts for about 5% of all Hodgkin lymphoma cases.
Hodgkin Lymphoma Drug Chapters
The drug chapter segment of the Hodgkin lymphoma report encloses a detailed analysis of the marketed and the late, mid, and early stage (Phase III, Phase II, and Phase I/II) pipeline drugs. The marketed drugs segment encloses drugs such as KEYTRUDA (Merck Sharp & Dohme), OPDIVO (Bristol Myers Squibb), and ADCENTRIS (Pfizer & Takeda). The drug chapter also helps understand the Hodgkin lymphoma clinical trial details, expressive pharmacological action, agreements and collaborations, approval and patent details, and the latest news and press releases.
Marketed Drugs
KEYTRUDA (pembrolizumab): Merck
It is an anti-PD-1 therapy that works by increasing the ability of the body’s immune system to help detect and fight tumor cells. KEYTRUDA is a humanized monoclonal antibody that blocks the interaction between PD-1 and its ligands, PD-L1 and PD-L2, thereby activating T lymphocytes which may affect both tumor cells and healthy cells.
In October 2020, the US Food and Drug Administration (FDA) approved an expanded label for KEYTRUDA, Merck’s anti-PD-1 therapy, as monotherapy for the treatment of adult patients with relapsed or refractory classical Hodgkin lymphoma.
It was approved under the FDA’s accelerated approval regulations, based on tumor response rate and durability of response. The approval is based on data from 210 patients in the KEYNOTE-087 trial, which showed an overall response rate (ORR) of 69 percent (95% CI: 62, 75) with KEYTRUDA (200 mg every three weeks), including a complete remission rate (CRR) of 22 percent and a partial remission rate (PRR) of 47 percent.
OPDIVO (nivolumab): Bristol Myers Squibb
Binding of the PD-1 ligands, PD-L1 and PD-L2, to the PD-1 receptor found on T cells, inhibits T-cell proliferation and cytokine production. Upregulation of PD-1 ligands occurs in some tumors and signaling through this pathway can contribute to inhibition of active T-cell immune surveillance of tumors. Nivolumab is a human immunoglobulin G4 (IgG4) monoclonal antibody that binds to the PD-1 receptor and blocks its interaction with PD-L1 and PD-L2, releasing PD-1 pathway-mediated inhibition of the immune response, including the anti-tumor immune response. In syngeneic mouse tumor models, blocking PD-1 activity resulted in decreased tumor growth.
In May 2016, the US FDA granted accelerated approval to OPDIVO (nivolumab) for the treatment of patients with classical Hodgkin lymphoma (cHL).
OPDIVO was approved prior to the Prescription Drug User Fee Act (PDUFA) goal date of September 1, 2016. It also received Breakthrough Therapy Designation for the treatment of relapsed or refractory classical Hodgkin lymphoma after failure of autologous hematopoietic stem cell transplant (HSCT) and brentuximab vedotin. Additionally, OPDIVO holds Orphan Drug status for the treatment of Hodgkin lymphoma. This application was granted Priority Review and approved under the FDA's Accelerated Approval Program.
ADCETRIS (brentuximab vedotin): Pfizer & Takeda
It is an antibody-drug conjugate (ADC) comprising an anti-CD30 monoclonal antibody attached by a protease-cleavable linker to a microtubule disrupting agent, monomethyl auristatin E (MMAE), utilizing Seagen’s proprietary technology. The ADC employs a linker system that is designed to be stable in the bloodstream but to release MMAE upon internalization into CD30-positive tumor cells. ADCETRIS has received marketing authorization by regulatory authorities in more than 70 countries for relapsed or refractory Hodgkin lymphoma and sALCL.
In November 2022, the FDA approved ADCETRIS, in combination with doxorubicin, vincristine, etoposide, prednisone, and cyclophosphamide for pediatric patients 2 years of age and older with previously untreated high-risk classical Hodgkin lymphoma. This marks the first pediatric approval for brentuximab vedotin.
|
Comparision of Marketed drugs | ||||
|
Product |
Company |
RoA |
MoA |
US Approval |
|
KEYTRUDA (pembrolizumab) |
Merck |
IV |
PD-1 inhibitor |
2017 |
|
OPDIVO (nivolumab) |
Bristol Myers Squibb |
IV |
PD-1 inhibitor |
2016 |
|
ADCETRIS (brentuximab vedotin) |
Pfizer & Takeda |
IV |
Delivering a cytotoxic molecule to CD30-positive cancer cells |
2011 |
Note: Detailed list will be provided in the final report.
Emerging Drugs
TEVIMBRA (tislelizumab): BeiGene
TEVIMBRA is a monoclonal antibody that is part of a drug class targeting either the PD-1 or PD-L1, which blocks the PD-1/PD-L1 pathway. This action removes the inhibition of the immune response, potentially disrupting peripheral tolerance and leading to immune-mediated adverse reactions.
In August 2024, BeinGene reported completion of Phase II clinical trial of tislelizumab in participants with relapsed or refractory classical Hodgkin lymphoma (TIRHOL).
Beigene also reported in March 2024, Phase II clinical trial of tislelizumab as first-line treatment of Hodgkin lymphoma for patients age 60 years and older, expected to complete in 2028.
Favezelimab/pembrolizumab: Merck
Favezelimab binds to LAG3 expressed on tumor-infiltrating lymphocytes (TILs) and blocks its binding with major histocompatibility complex (MHC) class II molecules expressed on tumor cells. Merck is developing a co-formulation of favezelimab/pembrolizumab for treatment of relapsed or refractory classical Hodgkin lymphoma and conducting a Phase III trial.
According to Phase I/II results presented in ASCO 2024, the ORR was 83% (n = 25; 95% CI, 65-94); 11 pts (37%) had a complete response and 14 (47%) had a partial response. Median DOR was 17.0 months (range, 2.6-30.5), and an estimated 47% of responders remained in response at 24 months. Median PFS was 19.4 months (95% CI, 9.5-28.5), and the 24-month PFS rate was 46%. With additional follow-up, favezelimab plus pembrolizumab continued to demonstrate sustained antitumor activity and manageable safety in pts with anti–PD-1–naive R/R cHL.
|
Comparison of Emerging Drugs Under Development for Hodgkin Lymphoma | ||||||
|
Product |
Company |
Mechanism of Action |
Phase |
Indication |
ROA |
Molecule Type |
|
Favezelimab/ pembrolizumab |
Merck |
LAG3 and PD-1 combination |
III |
Relapsed or refractory classical Hodgkin Lymphoma |
IV |
Monoclonal antibody |
|
TEVIMBRA (tislelizumab) |
BeiGene |
PD-1 inhibitor |
II |
Relapsed or refractory classical Hodgkin lymphoma |
IV |
Monoclonal antibody |
|
Autologous CD30 CAR-T cells |
Tessa Therapeutics |
Tumor cell inhibitor |
II |
Relapsed/Refractory Hodgkin Lymphoma |
IV |
CAR-T cell therapy |
|
GEN3017 |
Genmab |
CD30 and CD3 binder |
I/II |
Hodgkin lymphoma |
IV |
Bispecific monoclonal antibody |
|
Gemcitabine+ Bendamustine + Nivolumab |
Bristol-Myers Squibb |
DNA crosslinking inhibitor, PD-1 inhibitor |
I/II |
Relapsed or Refractory Classical Hodgkin Lymphoma |
IV |
Nucleoside analog, Alkylating agent, Immune checkpoint inhibitor |
|
AZD3470 |
AstraZeneca |
Protein arginine methyltransferase 5 inhibitor |
I/II |
Relapsed/Refractory Haematologic Malignancies |
Oral |
Small molecule |
|
Ipilimumab ± Nivolumab |
Bristol-Myers Squibb |
cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), PD-1 inhibitor |
II |
Relapsed or Refractory Classical Hodgkin Lymphoma |
IV |
Monoclonal antibody, Immune checkpoint inhibitor |
Note: Detailed list will be provided in the final report.
Drug Class Insights
The drug classes include cell death stimulants, CD30 and CD3 binders, and PD-L1 and PD-L2 antagonists. Additionally, there are tumor cell inhibitors and immune-modulating agents.
PD-1 inhibitors
Both PD-L1 and PD-L2 suppress T cell proliferation, cytokine production, and cell adhesion, although some data suggest they may also have a costimulatory role. Only PD-L2, however, triggers reverse signaling in dendritic cells, leading to IL-12 production and T cell activation, while PD-L1 does not. The expression of PD-L1 and PD-L2 is regulated by different stimuli, and their patterns suggest both shared and distinct roles in immune regulation. KEYTRUDA (pembrolizumab) and OPDIVO (nivolumab) both block the PD-1 receptor, preventing its interaction with PD-L1 and PD-L2, which boosts T cell responses against tumors. While their mechanisms are similar, OPDIVO may have a slightly higher binding affinity, which could influence its efficacy, dosing, side effect profile, and overall therapeutic effectiveness.
Note: Detailed insights will be provided in the final report.
Hodgkin Lymphoma Market Outlook
Hodgkin lymphoma is a cancer that forms in the lymph nodes, causing swelling in areas such as the neck, armpit, and groin. The growth of the market is primarily driven by the increasing number of special designations granted by regulatory authorities. Additionally, factors such as higher investments in healthcare infrastructure, the rising adoption of premium-priced products like immune checkpoint inhibitors, and government efforts to raise awareness in underserved regions are also contributing to this growth. Furthermore, the expanding potential in emerging economies and the growing demand for regenerative medicine are expected to create lucrative opportunities for market expansion. Strategic collaborations among market players are also playing a significant role in fostering market growth.
Hodgkin lymphoma is less common than non-Hodgkin lymphoma, typically affecting adults in two age groups: 20–39 and 65+. Over 75% of adults diagnosed with Hodgkin lymphoma are cured with standard treatments like chemotherapy and radiation. The drug ADCETRIS (brentuximab vedotin) targets CD30, a protein on Hodgkin lymphoma cells, and is approved for advanced cases. It may help older patients avoid toxic chemotherapy. Clinical trials are exploring its combination with other treatments. The drug is also approved for children and adolescents with Hodgkin lymphoma. Immune checkpoint inhibitors, such as OPDIVO (nivolumab) and KEYTRUDA (pembrolizumab) are effective for recurrent Hodgkin lymphoma and are being studied in combination with other therapies. Targeted immunotherapies, like SGN-35 (anti-CD30 antibody), show promise for both relapsed and newly diagnosed patients. Additionally, adoptive immunotherapy targeting EBV-associated antigens and biotoxins delivered to tumor-associated macrophages are being researched for more personalized treatment approaches.
To summarize, several new medicines are expected to enter the market for the treatment paradigm for Hodgkin lymphoma in the coming years. Demand for emerging therapies would be driven by physician enthusiasm, which a significant unmet need, frequent switching, and the usage of multi-drug regimens would fuel.
- Among the 7MM, the United States expected to capture the maximum market share in 2036 and expected to grow in the coming years.
- In the EU4 and the UK, Germany expected to capture the maximum share by 2036.
- Presently two PD-1 inhibitors namely KEYTRUDA and OPDIVO are approved and a new one TEVIMBRA is currently under Phase II of the clinical trial process for the treatment of relapsed or refractory classical Hodgkin lymphoma.
- Personalized therapies based on PET2 imaging have emerged as a promising strategy, showing impressive three-year progression-free survival (PFS) rates and supporting a more tailored, precise treatment approach.
- In June 2024, Takeda and Pfizer announced that the German Hodgkin Study Group (GHSG) would present positive results from the Phase III HD21 trial of ADCETRIS (brentuximab vedotin) combined with chemotherapy in late-breaking oral presentations at the 60th American Society of Clinical Oncology (ASCO) Annual Meeting (LBA7000) and the 29th European Hematology Association (EHA) Annual Meeting.
Hodgkin Lymphoma Drugs Uptake
This section focuses on the uptake rate of potential drugs expected to be launched in the market during 2020–2034. The landscape of Hodgkin lymphoma treatment has experienced a profound transformation with the uptake of novel drugs. These innovative therapies are redefining standards of care. Furthermore, the increased uptake of these transformative drugs is a testament to the unwavering dedication of physicians, oncology professionals, and the entire healthcare community in their tireless pursuit of advancing cancer care. This momentous shift in treatment paradigms is a testament to the power of research, collaboration, and human resilience.
Further detailed analysis of emerging therapies drug uptake in the report…
Hodgkin lymphoma Pipeline Development Activities
The report provides insights into therapeutic candidates in Phase III, Phase II, and Phase I/II. It also analyzes key players involved in developing targeted therapeutics.
Pipeline Development Activities
The report covers information on collaborations, acquisitions and mergers, licensing, and patent details for Hodgkin lymphoma emerging therapy.
KOL Views
To keep up with current market trends, we take KOLs and SMEs’ opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts contacted for insights on Hodgkin lymphoma evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake, along with challenges related to accessibility, including oncologists, radiation oncologists, surgical oncologists, and others.
Delveinsight’s analysts connected with 15+ KOLs to gather insights; however, interviews were conducted with 7+ KOLs in the 7MM. Centers such as - Memorial Sloan Kettering Cancer Center, Mount Sinai, Smilow Cancer Hospital Yale Cancer Center, National Institutes of Health in the USA, Moffitt Cancer Center, MD Anderson Cancer Center Madrid, Moffitt Cancer Center in Florida, University of Miami, etc., were contacted. Their opinion helps understand and validate current and emerging therapy treatment patterns or Hodgkin lymphoma market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
|
KOL Views |
|
“A multidisciplinary approach to Hodgkin lymphoma, which combines traditional and novel therapies, should be considered. The combination of nivolumab with chemotherapy has demonstrated superior results compared to standard regimens, indicating a shift toward more effective and personalized treatment strategies.” Professor Roger Williams Medical Center, United States |
|
“Treatment for Hodgkin lymphoma has improved significantly since the ABVD chemotherapeutic combination was invented over 30 years ago. Despite using the same ABVD regimen in most patients treated first line, we now have a much better understanding of disease biology, late side effects of therapy and have moved towards a personalized risk adapted approach.” MD, John Hopkins University, United States |
|
“The future of Hodgkin lymphoma treatment lies in personalized approaches that integrate these advanced therapies, providing hope for better long-term outcomes and improved quality of life for patients.” D.O., Northwestern University, United states |
Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT analysis and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of gaps in disease diagnosis, patient awareness, physician acceptability, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided.
Conjoint Analysis analyzes multiple approved and emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
In efficacy, the trial’s primary and secondary outcome measures are evaluated; for instance, in event- free survival, one of the most crucial primary outcome measures is event-free survival and overall survival.
Further, the therapies’ safety is evaluated, wherein the acceptability, tolerability, and adverse events are majorly observed, and this clearly explains the drugs side effects in the trials. In addition, the scoring is also based on the probability of success and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Market Access and Reimbursement
Reimbursement may be referred to as the negotiation of a price between a manufacturer and payer that allows the manufacturer access to the market. It is provided to reduce the high costs and make the essential drugs affordable. Health technology assessment (HTA) plays an important role in reimbursement decision-making and recommending the use of a drug. These recommendations vary widely throughout the seven major markets, even for the same drug.
In the US healthcare system, both Public and Private health insurance coverage are included. Also, Medicare and Medicaid are the largest government-funded programs in the US. The major healthcare programs including Medicare, Medicaid, the Children's Health Insurance Program (CHIP), and the state and federal health insurance marketplaces are overseen by the Centers for Medicare & Medicaid Services (CMS). Other than these, Pharmacy Benefit Managers (PBMs), and third-party organizations that provide services, and educational programs to aid patients are also present.
The report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of approved therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Further detailed analysis of emerging therapies drug uptake in the report…
Scope of the Report
- The report covers a segment of key events, an executive summary, and a descriptive overview of Hodgkin lymphoma, explaining its causes, signs, symptoms, pathogenesis, and currently used therapies.
- Comprehensive insight into the epidemiology segments and forecasts, disease progression, and treatment guidelines has been provided.
- Additionally, an all-inclusive account of the emerging therapies and the elaborative profiles of late-stage and prominent therapies will impact the current treatment landscape.
- A detailed review of the Hodgkin lymphoma market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help shape and drive the 7MM Hodgkin lymphoma market.
Hodgkin Lymphoma Report Insights
- Patient Population
- Therapeutic Approaches
- Hodgkin lymphoma Pipeline Analysis
- Hodgkin lymphoma Market Size and Trends
- Existing and Future Market Opportunity
Hodgkin Lymphoma Report Key Strengths
- Eleven-Year Forecast
- The 7MM Coverage
- Hodgkin Lymphoma Epidemiology Segmentation
- Key Cross Competition
- Drugs Uptake and Key Market Forecast Assumptions
Hodgkin Lymphoma Report Assessment
- Current Treatment Practices
- Unmet Needs
- Pipeline Product Profiles
- Market Attractiveness
- Qualitative Analysis (SWOT Analysis and Conjoint Analysis)
FAQs
- What was the Hodgkin lymphoma market size, the market size by therapies, market share (%) distribution in 2023, and what would it look like by 2036? What are the contributing factors for this growth?
- What are the pricing variations among different geographies for approved therapies?
- What can be the future treatment paradigm of Hodgkin lymphoma?
- What are the disease risks, burdens, and unmet needs of Hodgkin lymphoma? What will be the growth opportunities across the 7MM concerning the patient population with Hodgkin lymphoma?
- What are the current options for the treatment of Hodgkin lymphoma? What are the current guidelines for treating Hodgkin lymphoma in the US, Europe, and Japan?
- What are the recent novel therapies, targets, mechanisms of action, and technologies being developed to overcome the limitations of existing therapies?
Reasons to Buy
- The report will help develop business strategies by understanding the latest trends and changing treatment dynamics driving the Hodgkin lymphoma market.
- Insights on patient burden/disease prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- Understand the existing market opportunities in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identifying strong upcoming players in the market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the Analyst view section to provide visibility around leading classes.
- Highlights of access and reimbursement policies of current therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing market so that the upcoming players can strengthen their development and launch strategy.
-market.png&w=256&q=75)