Keratoconus Market
Key Highlights
- Keratoconus is one of the leading indications for corneal transplantation in Western countries and can be associated with other medical conditions. Keratoconus typically manifests during puberty or early adulthood, leading to irregular stromal thinning. This results in a cone-like bulge or protrusion and significant vision loss.
- In the US, keratoconus is most prevalent in individuals 18 to 39 years of age. The keratoconus prevalence is higher specifically in female individuals, and the diagnosis is often delayed in these patients.
- Keratoconus can be classified into three categories based on severity: mild, moderate, and severe. Assessments indicate that the combined prevalence of mild to moderate keratoconus is the highest.
- A positive family history or familial transmission is evident in at least 6–8% of reported cases. Among individuals with Down syndrome, the prevalence of keratoconus ranges from 0.5–15%.
- The treatment landscape has expanded significantly from conservative management (e.g., spectacles and contact lenses wear) and penetrating keratoplasty to many other therapeutic and refractive modalities, including corneal cross-linking (with various protocols/techniques), combined CXL-keratorefractive surgeries, intracorneal ring segments, anterior lamellar keratoplasty, and more recently, Bowman’s layer transplantation, stromal keratophakia, and stromal regeneration.
- Glaukos’ first-generation iLink therapy, known as PHOTREXA, or Epi-off, is the first and only FDA-approved therapy that has been shown to slow or halt disease progression.
- The emerging pipeline for keratoconus remains limited, with only a few investigational products in development, including Epioxa (Glaukos), EpiSmart (Epion Therapeutics), among others, aiming to refine and expand CXL capabilities.
- Ongoing innovations are primarily focused on minimally invasive delivery methods, enhancing patient comfort, accelerating recovery, and improving visual rehabilitation. In summary, the keratoconus market is expected to evolve with the introduction of new therapies in the coming years.
DelveInsight’s "Keratoconus – Market Insight, Epidemiology, and Market Forecast – 2034" report delivers an in-depth understanding of keratoconus, historical and forecasted epidemiology, as well as the keratoconus market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
Keratoconus market report provides real-world prescription pattern analysis, emerging drugs assessment, market share, and uptake/adoption pattern of individual therapies, as well as historical and forecasted keratoconus market size from 2020 to 2034 in 7MM. The report also covers current keratoconus treatment practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s underlying potential.
|
Study Period |
Geography Covered
- The United States
- EU4 (Germany, France, Italy, and Spain) and the United Kingdom
- Japan
Study Period: 2020–2034
Keratoconus Disease Understanding and Treatment Algorithm
Keratoconus Overview
Keratoconus, also known as conical cornea, is a progressive, non-inflammatory disorder characterized by thinning and bulging of the cornea into a cone-like shape. This deformation leads to irregular astigmatism, blurred or distorted vision, glare, and light sensitivity. Typically emerging during puberty or the late teens, keratoconus can progress for 10–20 years before stabilizing. Each eye may be affected differently. In advanced cases, sudden corneal swelling (acute hydrops) may occur due to a break in the Descemet’s membrane, causing abrupt vision loss. This swelling gradually resolves but may leave scar tissue. The condition is classified into subtypes based on the cone's shape and location—nipple, oval, D-shaped, and keratoglobus forms. While the exact cause remains unclear, genetic predisposition, environmental factors, and chronic eye rubbing are considered potential contributors.
Keratoconus Diagnosis
Keratoconus is diagnosed through a combination of clinical evaluation and advanced corneal imaging. Slit-lamp examination can reveal characteristic signs such as corneal thinning, Fleischer rings, Vogt’s striae, and apical scarring. Corneal topography is the gold standard for detecting early, subtle changes by mapping curvature and identifying asymmetric steepening, while corneal tomography offers a 3D assessment of both anterior and posterior corneal surfaces. Pachymetry helps confirm localized thinning, and keratometry measures curvature steepness. Early detection through these methods is essential to initiate timely intervention and prevent significant vision loss.
Further details related to diagnosis will be provided in the report…
Keratoconus Treatment
Treatment of keratoconus focuses on improving vision and slowing disease progression. In early stages, prescription glasses or soft contact lenses may correct mild refractive errors. As the condition advances, rigid gas permeable, scleral, or hybrid contact lenses are often used to provide a smoother optical surface. To halt progression, corneal collagen cross-linking (CXL) is the only proven intervention, strengthening corneal tissue and reducing further deformation. In cases of significant scarring or advanced thinning where lenses no longer provide adequate vision, surgical options such as intracorneal ring segments (ICRS), deep anterior lamellar keratoplasty (DALK), or penetrating keratoplasty (full-thickness corneal transplant) may be required. Supportive measures, including avoidance of eye rubbing and management of allergies, are also recommended to reduce the risk of worsening.
Further details related to treatment will be provided in the report…
Keratoconus Epidemiology
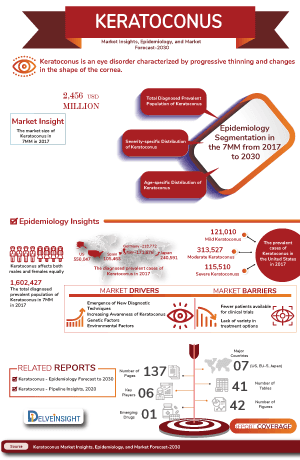
The keratoconus epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by the total prevalent cases of keratoconus, severity-specific cases of keratoconus, age-specific cases of keratoconus, gender-specific cases of keratoconus in the 7MM market covering the United States, EU4 (Germany, France, Italy, and Spain), and the United Kingdom, and Japan from 2020 to 2034.
- In the US, the prevalence of keratoconus is approximately 138 per 100,000 individuals.
- The condition affects between 50 and 230 individuals per 100,000 in the general population. Keratoconus usually begins in adolescence or early adulthood and tends to progress until the fourth decade of life.
- While keratoconus is more commonly diagnosed in younger individuals, studies do show a prevalence between 7.4% and 15% in the over-50 age group.
- After adjusting for age, females had a hazard ratio of 1.295, indicating nearly 30% higher risk than males.
- Pediatric populations have a higher prevalence rate, with a reported prevalence rate ranging from 5.2 per 1,000 people.
Keratoconus Drug Chapters
Marketed Drugs
PHOTREXA (Riboflavin topical): Glaukos Corporation/Avedro
PHOTREXA VISCOUS/PHOTREXA are photoenhancers indicated for use with the KXL System in corneal collagen cross-linking for the treatment of progressive keratoconus and corneal ectasia following refractive surgery. It is the only FDA-approved bioactivated riboflavin ophthalmic solution. Glaukos’ first-generation iLink therapy, known as PHOTREXA, or Epi-off, is the first and only FDA-approved therapy that has been shown to slow or halt disease progression.
Emerging Drugs
Epioxa (Epi-on): Glaukos
EPIOXA, which is designed to preserve the corneal epithelium, reduce procedure times, improve patient comfort, and shorten recovery time, utilizes a proprietary, novel drug formulation designed to penetrate the epithelial layer of the cornea, a stronger UV-A irradiation protocol and supplemental oxygen to enhance cross-linking. If approved, the company anticipates EPIOXA would be the first FDA-approved, non-invasive corneal cross-linking therapy that does not require removal of the corneal epithelium, the outermost layer of the front of the eye.
Following the submission of the New Drug Application (NDA) for Epioxa, the FDA has set a Prescription Drug User Fee Act (PDUFA) goal date of October 20, 2025, for completion of its review. This reflects a standard 10-month review period, aligning with the company’s expectations.
EpiSmart: Epion Therapeutics
EpiSmart is an investigational drug-device combination product. EpiSmart and its components (Epiprep, Ribostat, and UVA Device) are not yet approved by the Food and Drug administration or by other regulatory bodies. It is in Phase III of development in patients 8 to 45 years of age with keratoconus.
Epion has developed a drug, drug delivery devices, and a UVA light device that fully optimize cross-linking and allow for the minimally-invasive treatment for corneal ectasias, including keratoconus. Unlike current treatments for keratoconus, EpiSmart is designed to be implemented upon initial diagnosis and can prevent disease progression. Epion’s elegant approach elevates the current standard of care for keratoconus by enabling the treatment of both eyes simultaneously and eliminating the need for further deterioration.
Note: A Detailed therapy assessment will be provided in the final report.
Drug Class Insight
In the marketed class of drugs, the cornerstone of therapy includes a photosensitizer such as PHOTREXA.
Photosensitizer
A photosensitizer works by using a light-activated molecule to trigger targeted chemical reactions. In the case of riboflavin in PHOTREXA, the photosensitizer absorbs UVA light at a wavelength of around 365–370 nm, becoming excited to a higher energy state. This energy is then transferred to oxygen molecules within the corneal tissue, producing reactive oxygen species (ROS) such as singlet oxygen and superoxide anions. These ROS initiate oxidative reactions that create additional covalent cross-links between collagen fibrils in the corneal stroma. As a result, the cornea becomes biomechanically stronger and more resistant to further deformation, helping to halt the progression of keratoconus and corneal ectasia.
Keratoconus Market Outlook
The therapeutic landscape for keratoconus is well-established, with current management focused on vision correction and slowing disease progression. Corneal collagen cross-linking (CXL) remains the only proven intervention to halt progression, with riboflavin-based products such as PHOTREXA approved for clinical use. Vision rehabilitation options range from glasses and soft contact lenses in early disease to rigid gas permeable, scleral, or hybrid lenses in advanced stages. Surgical interventions, including intracorneal ring segments (ICRS) and corneal transplantation, are reserved for severe or unresponsive cases. While pharmacological options remain limited, the emerging pipeline includes investigational agents such as Epioxa (a topical cross-linking formulation) and other next-generation CXL systems designed to enhance efficacy, reduce treatment time, and improve safety. Innovation in the field is largely centered on optimizing cross-linking techniques, developing minimally invasive delivery methods, and advancing custom lens technologies rather than introducing entirely new molecular targets.
Further details will be provided in the report….
Keratoconus Drugs Uptake
This section focuses on the uptake rate of potential drugs expected to be launched in the market during 2025–2034. The landscape of keratoconus treatment has experienced a profound transformation with the uptake of novel drugs.
Keratoconus Pipeline Development Activities
The report provides insights into different therapeutic candidates in mid and early stages. It also analyzes key players involved in developing targeted therapeutics.
Pipeline Development Activities
The report covers detailed information on collaborations, acquisitions and mergers, licensing, and patent details for keratoconus emerging therapies.
KOL- Views
To keep up with current market trends, we take KOLs and SMEs' opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Some of the leaders like MD, Professor and Vice Chair of the Department of Medicine and Director, PhD, and others. Their opinion helps to understand and validate current and emerging therapies and treatment patterns or keratoconus market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
Delveinsight’s analysts connected with 15+ KOLs to gather insights; however, interviews were conducted with 5+ KOLs in the 7MM. Centers such as the Washington University School of Medicine, University Medical Center Hamburg-Eppendorf, and University Graduate School of Medicine etc. were contacted. Their opinion helps understand and validate keratoconus epidemiology and market trends.
Qualitative Analysis
We perform qualitative and market intelligence analysis using various approaches, such as SWOT and conjoint analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Conjoint Analysis analyzes multiple approved and emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, designation, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
The analyst analyzes multiple emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry.
In efficacy, the trial’s primary and secondary outcome measures are evaluated.
Further, the therapies’ safety is evaluated, wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials.
Market Access and Reimbursement
Reimbursement may be referred to as the negotiation of a price between a manufacturer and a payer that allows the manufacturer access to the market. It is provided to reduce the high costs and make the essential drugs affordable. Health technology assessment (HTA) plays an important role in reimbursement decision-making and recommending the use of a drug. These recommendations vary widely throughout the seven major markets, even for the same drug. In the US healthcare system, both Public and Private health insurance coverage are included. Also, Medicare and Medicaid are the largest government-funded programs in the US. The major healthcare programs, including Medicare, Medicaid, Health Insurance Program (CHIP), and the state and federal health insurance marketplaces, are overseen by the Centers for Medicare & Medicaid Services (CMS). Other than these, Pharmacy Benefit Managers (PBMs) and third-party organizations that provide services and educational programs to aid patients are also present.
The report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of currently used therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Further detailed analysis will be provided in the report….
Scope of the Report
- The report covers a descriptive overview of keratoconus, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight has been provided into keratoconus epidemiology and treatment.
- Additionally, an all-inclusive account of the current therapies for keratoconus is provided, along with the assessment of new therapies, which will have an impact on the current treatment landscape.
- A detailed review of the keratoconus market, historical and forecasted, is included in the report, covering the 7MM drug outreach.
- The report provides an edge while developing business strategies, by understanding trends shaping and driving the 7MM keratoconus market.
Keratoconus Report Insights
- Patient Population
- Therapeutic Approaches
- Keratoconus Pipeline Analysis
- Keratoconus Market Size and Trends
- Market Opportunities
- Impact of Upcoming Therapies
Keratoconus Report Key Strengths
- Ten-Year Forecast
- 7MM Coverage
- Keratoconus Epidemiology Segmentation
- Key Cross Competition
- Highly Analyzed Market
- Drugs Uptake
Keratoconus Report Assessment
- Current Treatment Practices
- Unmet Needs
- Analyst Views
- Pipeline Product Profiles
- Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
FAQs
- What was the keratoconus market share (%) distribution in 2020, and what would it look like in 2034?
- What would be the keratoconus total market size, as well as market size by therapies across the 7MM during the study period (2020–2034)?
- What are the key findings about the market across the 7MM, and which country will have the largest keratoconus market size during the study period (2020–2034)?
- At what CAGR, the keratoconus market expected to grow at the 7MM level during the study period (2020–2034)?
- What would be the keratoconus market growth till 2034?
- What are the disease risks, burdens, and unmet needs of keratoconus?
- What is the historical keratoconus patient pool in the United States, the EU4 (Germany, France, Italy, and Spain), the UK, and Japan?
- What will be the growth opportunities across the 7MM concerning the patient population of keratoconus?
- Among the 7MM, which country would have the most prevalent cases of keratoconus?
- At what CAGR is the population expected to grow across the 7MM during the study period (2020–2034)?
- How many companies are developing therapies for the treatment of keratoconus?
- What are the key collaborations (industry–industry, industry-academia), mergers and acquisitions, and licensing activities related to keratoconus therapies?
- What are the recent novel therapies, targets, mechanisms of action, and technologies developed to overcome the limitations of existing therapies?
- What are the 7MM historical and forecasted market of keratoconus?
Reasons to buy
- The report will help in developing business strategies by understanding trends shaping and driving the keratoconus market.
- To understand the future market competition in the keratoconus market and insightful review of the SWOT analysis of keratoconus.
- Organize sales and marketing efforts by identifying the best opportunities for keratoconus in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identification of strong upcoming players in the market will help in devising strategies that will help in getting ahead of competitors.
- To understand the future market competition in keratoconus.