Metastatic Colorectal Cancer Market Summary
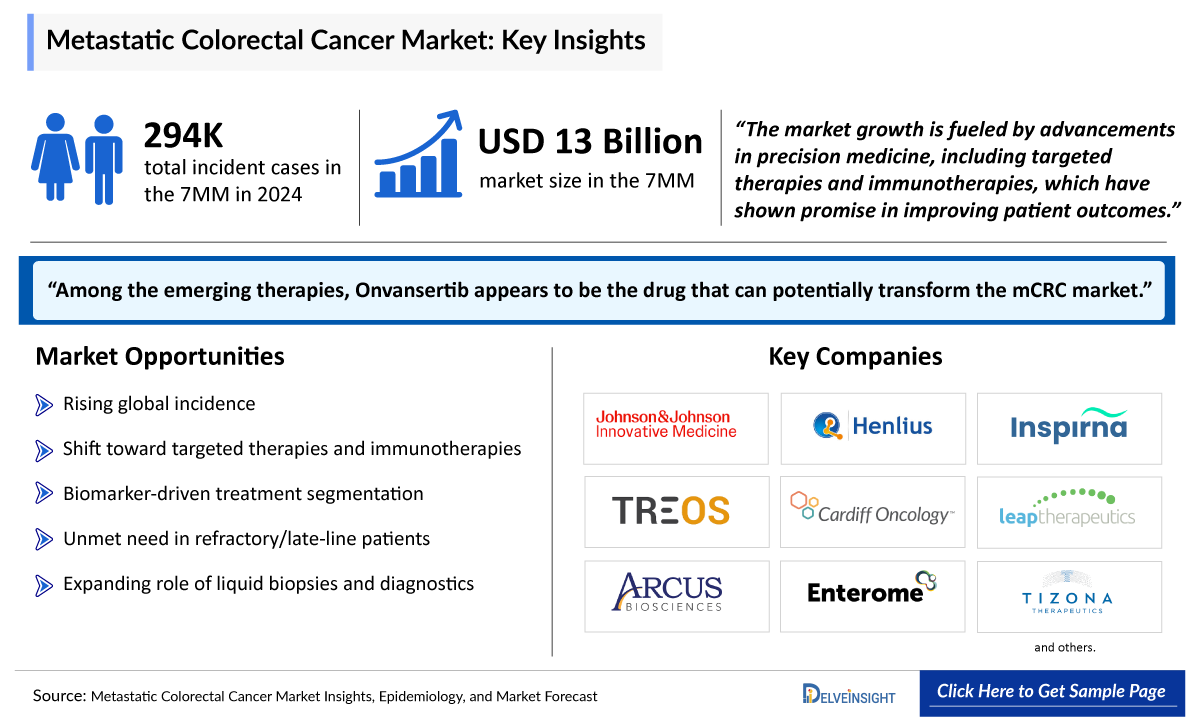
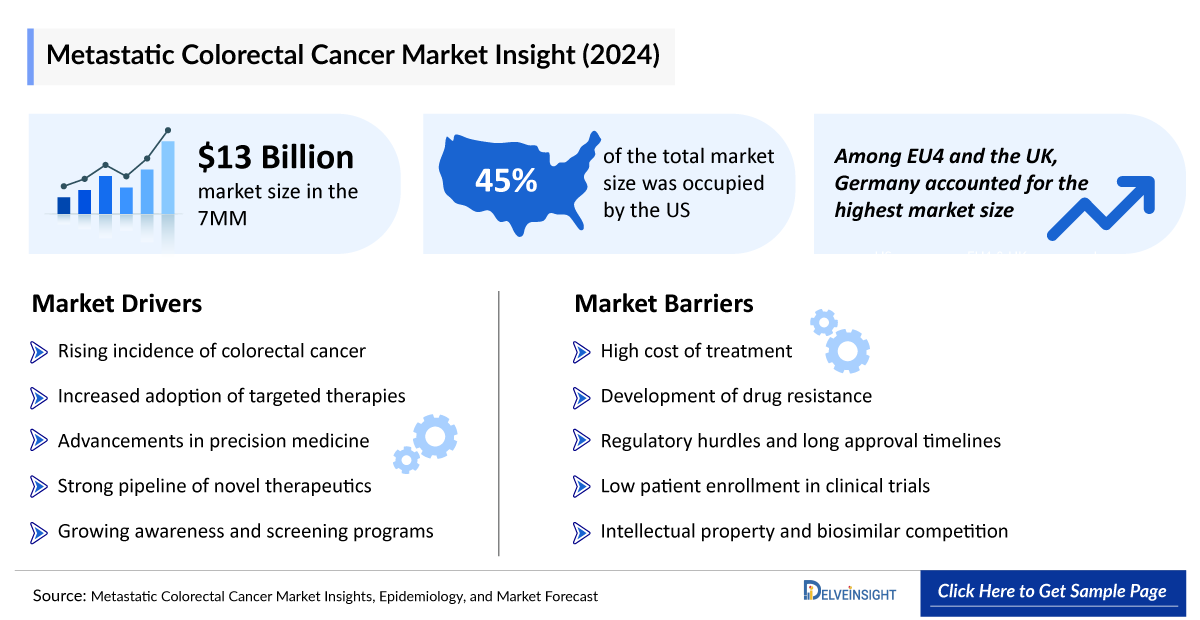
- In 2024, the total market size of mCRC in the 7MM was more than USD 13,000 million, which is expected to increase by 2034, during the study period (2020–2034).
- The United States accounted for the highest market size among the 7MM around 45% of the total market size.
- Among EU4 and the UK, Germany accounted for the highest market size in 2024, while Spain accounted for the lowest market size.
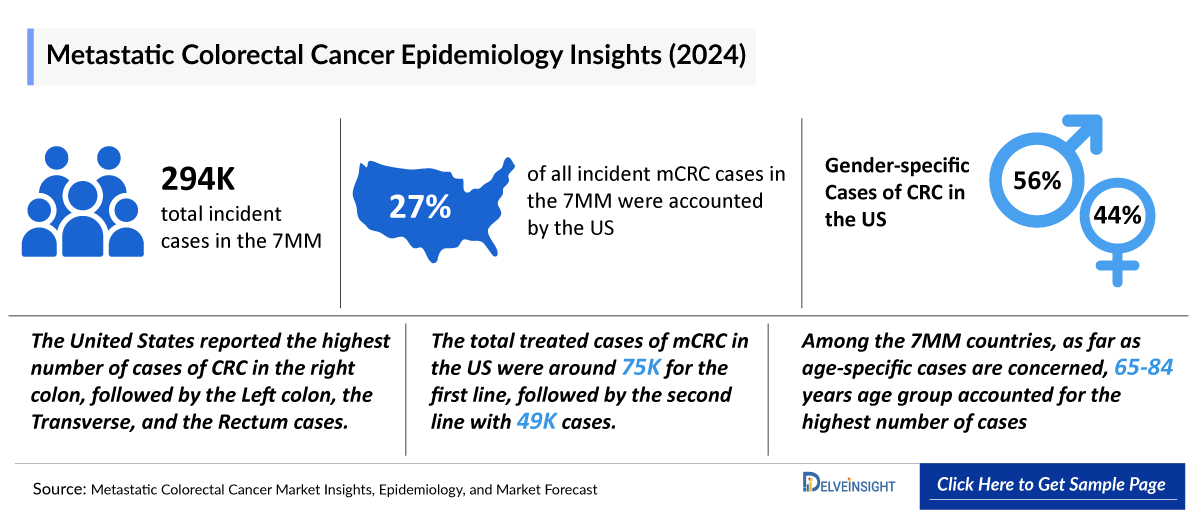
- In 2024, the incident cases of mCRC were approximately 294,500 cases in the 7MM, which will increase by 2034. In the 7MM, the highest number of incident cases of mCRC were observed in the US.
- In the US, Males were more affected by CRC than Females, in 2024.
- In 2024, the incident cases of mCRC were approximately 79,000 cases in the 7MM, which will increase by 2034.
- Among EU4 and the UK, in 2024, Germany accounted for the highest number of mCRC cases, followed by Italy, whereas Spain accounted for the lowest number of incident cases.
- The major sites of tumor localization in CRC are the right colon, transverse, left colon, rectosigmoid, and rectum.
- The diagnosis of mCRC involves a combination of clinical evaluation, imaging, and molecular testing to confirm disease spread and guide treatment. Contrast-enhanced CT scans of the chest, abdomen, and pelvis are standard for detecting metastases, with MRI for liver involvement and PET scans in select cases. Colonoscopy with biopsy confirms malignancy, while molecular testing for RAS (KRAS/NRAS), BRAF, MSI/MMR status, and HER2 or NTRK alterations informs targeted therapy selection.
- After someone is diagnosed with CRC, staging is done to figure out if it has spread, and if so, how far. CRC can be divided into localized, regional, distant, and unknown based on CRC staging.
- The primary therapy for resectable CRC is surgical removal, and in non-resectable CRC, standard therapies include chemotherapy, radiotherapy, and immunotherapy. However, these therapies have certain drawbacks, such as being non-specific and cytotoxic to normal cells, which leads to secondary complications.
- The treatment landscape for mCRC follows a multi-line approach. First-line (1L) therapy includes multi-agent chemotherapy (FOLFOX, FOLFIRI, CAPEOX), anti-angiogenesis agents (± chemotherapy), and targeted therapies like VECTIBIX and ERBITUX (± chemotherapy). Additionally, KEYTRUDA and BRAFTOVI (with ERBITUX and mFOLFOX6) are approved in 1L. Second-line (2L) options expand on 1L regimens with OPDIVO (± YERVOY), JEMPERLI, TUKYSA (± Trastuzumab), LONSURF (± Bevacizumab), and KRAZATI + ERBITUX. Third-line (3L) therapy includes STIVARGA and FRUZAQLA as specific options.
Metastatic Colorectal Cancer Market Report Summary
- The Metastatic Colorectal Cancer market report offers extensive knowledge regarding the epidemiology segments and predictions, presenting a deep understanding of the potential future growth in diagnosis rates, disease progression, and treatment guidelines. It provides comprehensive insights into these aspects, enabling a thorough assessment of the subject matter.
- Additionally, an all-inclusive account of the current management techniques and emerging therapies and the elaborative profiles of late-stage (Phase III and Phase II) and prominent therapies that would impact the current treatment landscape and result in an overall market shift has been provided in the report.
- The report also encompasses a comprehensive analysis of the mCRC market, providing an in-depth examination of its historical and projected market size (2020–2034). It also includes the market share of therapies, detailed assumptions, and the underlying rationale for our methodology. The report also includes drug outreach coverage in the 7MM region.
- The report includes qualitative insights that provide an edge while developing business strategies by understanding trends through SWOT analysis and expert insights/KOL views, including experts from various hospitals and prominent universities, patient journey, and treatment preferences that help shape and drive the 7MM mCRC market.
The table given below further depicts the key segments provided in the report:
Factors impacting the Metastatic Colorectal Cancer (mCRC) Market Growth
Metastatic Colorectal Cancer incidence fueling demand
Colorectal cancer is the second leading cause of cancer mortality. In 2024, approximately 170K metastatic cases were reported across the 7MM. Despite screening improvements, aging populations and lifestyle factors are expected to sustain incidence by 2034.
Metastatic Colorectal Cancer treatment paradigm
Current approaches involve chemotherapy (FOLFOX, FOLFIRI) with targeted agents (bevacizumab, cetuximab, panitumumab). Immunotherapy has transformed MSI-H/dMMR subsets, but RAS-mutant and MSS patients continue to face poor outcomes.
Metastatic Colorectal Cancer pipeline spotlight
Key innovations include adagrasib (BMS) and sotorasib (Amgen) for KRAS G12C-mutant tumors, bispecific antibodies (REGN1979), and ADC candidates (Seagen, Daiichi Sankyo). Novel IO combinations (PD-1 + VEGF blockade) are also gaining traction.
Metastatic Colorectal Cancer (mCRC) Market
- Various key players are leading the treatment landscape of mCRC, such as Takeda Pharmaceuticals, Bristol-Myers Squibb, Merck, Amgen, and Others. The details of the country-wise and therapy-wise market size have been provided below.
- In the total market size of mCRC in the 7MM, the United States accounted for the highest market share, i.e. ~45% in 2024, followed by Japan.
- Among EU4 and the UK, Germany accounted for the highest market size in 2024.
- The United States accounted for more than USD 6,000 million in 2024.
- Among the emerging therapies, Onvansertib appears to be the drug that can potentially transform the mCRC market.
Metastatic Colorectal Cancer (mCRC) Recent Developments and Breakthroughs
- In April 2025, Biocon Biologics received FDA approval for Jobevne™ (bevacizumab-nwgd), a biosimilar to Avastin®, for intravenous use. Jobevne is approved for multiple cancers, including metastatic colorectal cancer, non-squamous non-small cell lung cancer, recurrent glioblastoma, metastatic renal cell carcinoma, advanced cervical cancer, and ovarian cancer, fallopian tube cancer, or primary peritoneal cancer.
- In April 2025, the FDA approved nivolumab (Opdivo) combined with ipilimumab (Yervoy) for adults and pediatric patients (12+) with unresectable or metastatic MSI-H or dMMR colorectal cancer. The FDA also granted full approval to single-agent nivolumab for MSI-H or dMMR metastatic CRC patients aged 12 and older whose disease progressed after standard chemotherapy.
Metastatic Colorectal Cancer (mCRC) Drug Analysis
The section dedicated to drugs in the mCRC report provides an in-depth evaluation of late-stage pipeline drugs (Phase III and Phase II) related to mCRC. The drug chapters section provides valuable information on various aspects related to clinical trials of mCRC, such as the pharmacological mechanisms of the drugs involved, designations, approval status, patent information, and a comprehensive analysis of the pros and cons associated with each drug. Furthermore, it presents the most recent news updates and press releases on drugs targeting mCRC.
Metastatic Colorectal Cancer Marketed Therapies
FRUZAQLA (fruquintinib): Takeda Pharmaceuticals
FRUZAQLA (fruquintinib) is an oral, once-a-day capsule developed by Takeda Pharms. It is a kinase inhibitor approved for the treatment of adult patients with metastatic Colorectal Cancer (mCRC) who have previously received fluoropyrimidine-, oxaliplatin-, irinotecan-based chemotherapy, an anti-VEGF therapy, and, if appropriate, an anti-EGFR therapy for RAS wild-type patients. It works by selectively inhibiting Vascular Endothelial Growth Factor Receptor (VEGFR)-1, -2, and -3, blocking VEGF-induced endothelial cell proliferation and tumor growth.
FRUZAQLA was approved by the FDA in November 2023 for previously treated mCRC, by the European Commission in June 2024 for the same indication, and by Japan in September 2024 as the first novel targeted therapy for mCRC in over a decade.
OPDIVO QVANTIG (nivolumab and hyaluronidase-nvhy): Bristol Myers Squibb
OPDIVO QVANTIG (nivolumab and hyaluronidase-nvhy) injection is a treatment for adult patients with MSI-H or dMMR mCRC that has progressed after treatment with a fluoropyrimidine, oxaliplatin, and irinotecan, either as monotherapy or following combination treatment with intravenous nivolumab and ipilimumab. It works by binding to the PD-1 receptor, blocking its interaction with PD-L1 and PD-L2.
In December 2024, the FDA approved OPDIVO QVANTIG for subcutaneous injection in the treatment of mCRC. With this approval, it is now the first and only subcutaneously administered PD-1 inhibitor, offering a faster delivery for patients to receive this immunotherapy treatment option in 3–5 min compared to 30-min IV OPDIVO.
Metastatic Colorectal Cancer Emerging Therapies
RYBREVANT (amivantamab) + Chemotherapy: Johnson & Johnson Innovative Medicine
Amivantamab is a bispecific antibody that exhibits specific genetic mutations, particularly EGFR exon 20 insertion mutations. Its mechanism of action involves dual targeting of the epidermal Growth Factor Receptor (eGFR) and the MET receptor, which are critical in tumor growth and survival. By binding to these receptors, amivantamab disrupts their signaling pathways, effectively overcoming certain resistance mechanisms that tumors may develop against other therapies.
This action not only inhibits cancer cell proliferation but also enhances the immune response against the tumor, leading to increased cancer cell death. Currently, the drug is in the Phase III stage of its clinical trial for the treatment of mCRC.
Zanzalintinib (XL092): Exelixis
Zanzalintinib (XL-092) is a third-generation oral tyrosine kinase inhibitor that inhibits the activity of receptor tyrosine kinases implicated in cancer growth and spread, including VEGF receptors, MET, AXL, and MER. These receptor tyrosine kinases are involved in both normal cellular function and in pathologic processes such as oncogenesis, metastasis, tumor angiogenesis, and resistance to multiple therapies, including immune checkpoint inhibitors.
With Zanzalintinib, Exelixis sought to build upon its extensive experience with the target profile of cabozantinib, the company’s flagship medicine, while improving key characteristics, including pharmacokinetic half-life. Currently, the drug is in the Phase III stage of its clinical trial for the treatment of mCRC.
Metastatic Colorectal Cancer (mCRC) Market Outlook
The metastatic colorectal cancer (mCRC) treatment landscape has evolved significantly, with multiple targeted therapies, immunotherapies, and novel agents expanding beyond traditional chemotherapy. First-line treatment relies on multiagent chemotherapy regimens like FOLFOX, FOLFIRI, and CAPEOX, often combined with anti-angiogenesis or EGFR inhibitors such as bevacizumab, cetuximab, and panitumumab, depending on molecular profiling. Recent approvals have introduced precision medicines like FRUZAQLA, KRAZATI, and LUMAKRAS, addressing specific genetic mutations such as KRAS G12C and BRAF V600E, while immunotherapies like KEYTRUDA, OPDIVO, and JEMPERLI continue to shape the MSI-H/dMMR treatment space. However, despite these advancements, the market remains highly competitive, with significant unmet needs for durable and long-term treatment responses. Ongoing research into investigational agents like amivantamab, zanzalintinib, and vactosertib highlights the push for more effective, resistance-targeting, and tumor microenvironment-modifying therapies, signaling a dynamic and evolving future for mCRC treatment.
The US has the highest market share for mCRC, followed by Japan, and then Germany. Rising awareness about the disease and supporting government policies, funds, approvals, and emerging research would drive the market significantly in forecast periods (2025–2034).
Further details are provided in the report…
Metastatic Colorectal Cancer (mCRC) Disease Understanding
Metastatic Colorectal Cancer (mCRC) Overview
Colorectal cancer (CRC) is the third most common, with metastasis being the major cause of death in the majority of patients. Common sites of distant metastasis are the liver and the peritoneum. CRC starts in the colon or the rectum. These cancers can also be called colon cancer or rectal cancer, depending on where they start. Colon cancer and rectal cancer are often grouped because they have many features in common. CRC may develop when polyps, mushroom-like growths inside the colon, grow and become cancerous, or cells along the lining of the colon or rectum mutate and grow out of control, forming a tumor.
CRC that spreads, or metastasizes, to the lungs, liver, or any other organ is called metastatic colorectal cancer (mCRC). The most common site of metastases for colon or rectal cancer is the liver. CRC cells may also spread to the lungs, bones, brain, or spinal cord.
Further details are provided in the report…
Metastatic Colorectal Cancer (mCRC) Diagnosis
Patients can present with a wide range of signs and symptoms such as occult or overt rectal bleeding, change in bowel habits, anemia, or abdominal pain. However, CRC is largely an asymptomatic disease until it reaches an advanced stage. By contrast, rectal bleeding is a common symptom of both benign and malignant causes. Therefore additional risk factors might be needed to help identify those people who should undergo further investigation by colonoscopy. New-onset rectal bleeding should generally prompt colonoscopy in individuals aged 45 years or older. In younger patients, additional factors are used to identify those at the highest risk for CRC (e.g., having a family history of CRC, change in bowel habits, unexplained weight loss, and blood mixed with the stool as opposed to blood on the surface of the stool).
Further details related to country-based variations are provided in the report…
Metastatic Colorectal Cancer (mCRC) Treatment
Metastatic CRC is still an incurable disease for most patients, with most commonly liver, lung, or lymph nodes and peritoneal metastases. In the past, 15 years ago, the median overall survival (mOS) was approximately 12 months, and the 5-year survival rate was 13%. However, the survival rate of these patients has increased, mainly due to the combined treatment of metastases with surgery and systemic therapy. Long-term survival or even cure can be attained of the patients who undergo complete R0 resection of liver or lung metastases, and 5-year survival of these patients can be achieved.
However, in the field of systemic therapy, there has been significant progress with new drugs in recent years. There are more options of initial systemic chemotherapy, oxaliplatin, irinotecan, and fluoropyrimidines, in combination with targeted therapy with anti-epidermal growth factor receptor (EGFR) monoclonal antibodies (cetuximab, panitumumab) in case of KRAS wild type tumors or anti-vascular endothelial growth factor (VEGF) inhibitors (monoclonal antibodies bevacizumab, aflibercept, ramucirumab, regorafenib as per oral tyrosine kinase inhibitor). The combination of these novel chemotherapies and targeted therapy now extends the mOS up to 40 months.
Further details related to treatment and management are provided in the report…
Metastatic Colorectal Cancer (mCRC) Epidemiology
The mCRC epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by total incident cases of CRC, gender-specific cases of CRC, age-specific cases of CRC, tumor location-specific cases of CRC, stage-specific cases of CRC, total incident cases of mCRC, mutation type-specific cases of mCRC, and total treated cases of mCRC in the United States, EU4 countries (Germany, France, Italy, Spain) and the United Kingdom, and Japan from 2020 to 2034.
- The total incident cases of mCRC in the US were around 79,000 cases in 2024.
- In 2024, Gender-specific cases of CRC in the United States were around 85,000 and 67,000 for male and female, respectively.
- In 2024, the US recorded a higher Incidence of CRC cases in the 65-84 years age group, with approximately 68,000 cases, followed by the 45-64 years age group, which accounted for approximately 57,500 cases.
- In 2024, the United States reported the highest number of cases of CRC in the right colon followed by Left colon, Transverse, and Rectum cases.
- Germany had the highest incidence of mCRC among the EU4 and the UK, accounting for more than 33,000 cases in 2024.
- In 2024, the total treated cases of mCRC in the US were around 75,000 for the first line, followed by the second line with 49,000 cases.
Latest KOL Views on Metastatic Colorectal Cancer (mCRC)
To stay abreast of the latest trends in the market, we conduct primary research by seeking the opinions of Key Opinion Leaders (KOLs) and Subject Matter Experts (SMEs) who work in the relevant field. This helps us fill any gaps in data and validate our secondary research.
We have reached out to industry experts to gather insights on various aspects of mCRC, including the evolving treatment landscape, patients’ reliance on conventional therapies, their acceptance of therapy switching, drug uptake, and challenges related to accessibility. The experts we contacted included medical/scientific writers, professors, and researchers from prestigious universities in the US, Europe, the UK, and Japan.
Our team of analysts at Delveinsight connected with more than 15 KOLs across the 7MM. We contacted institutions such as the University of Tsukuba, Duke University, University of Glasgow, Washington University School of Medicine, etc., among others. By obtaining the opinions of these experts, we gained a better understanding of the current and emerging treatment patterns in the mCRC market, which will assist our clients in analyzing the overall epidemiology and market scenario.
Metastatic Colorectal Cancer Report Qualitative Analysis
We perform Qualitative and Market Intelligence analysis using various approaches, such as SWOT analysis and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Conjoint Analysis analyzes multiple approved and emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, designation, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy. In efficacy, the trial’s primary and secondary outcome measures are evaluated; for instance, in trials for mCRC, one of the most important primary endpoints was achieving LDH percentage change, transfusion avoidance, etc. Based on these, the overall efficacy is evaluated.
Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the route of administration, order of entry and designation, probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Metastatic Colorectal Cancer Market Access and Reimbursement
Because newly authorized drugs are often expensive, some patients escape receiving proper treatment or use off-label, less expensive prescriptions. Reimbursement plays a critical role in how innovative treatments can enter the market. The cost of the medicine, compared to the benefit it provides to patients who are being treated, sometimes determines whether or not it will be reimbursed. Regulatory status, target population size, the setting of treatment, unmet needs, the number of incremental benefit claims, and prices can all affect market access and reimbursement possibilities.
The report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of approved therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Metastatic Colorectal Cancer (mCRC) Report Insights
- Patient Population
- Therapeutic Approaches
- mCRC Market Size and Trends
- Existing Market Opportunity
Metastatic Colorectal Cancer (mCRC) Report Key Strengths
- Ten-year Forecast
- The 7MM Coverage
- mCRC Epidemiology Segmentation
- Key Cross Competition
Metastatic Colorectal Cancer (mCRC) Report Assessment
- Current Treatment Practices
- Reimbursements
- Market Attractiveness
- Qualitative Analysis (SWOT, Conjoint Analysis, Unmet needs)
Key Questions Answered in the Metastatic Colorectal Cancer Report
- Would there be any changes observed in the current treatment approach?
- Will there be any improvements in mCRC management recommendations?
- Would research and development advances pave the way for future tests and therapies for mCRC?
- Would the diagnostic testing space experience a significant impact and lead to a positive shift in the treatment landscape of mCRC?
- What kind of uptake will the new therapies witness in the coming years in mCRC patients?
Uncover More Information with Related Content:
- KRYSTAL-1: A pooled phase 1/2 efficacy and safety of adagrasib (MRTX849) in combination with cetuximab in patients with metastatic colorectal cancer (CRC) harboring a KRASG12C mutation
- Novel and Emerging Metastatic Colorectal Cancer Treatment Drugs Anticipated to Change Market Dynamics
- Unlocking New Avenues in KRAS-Driven Cancer Research Beyond G12C
- BeiGene’s Brukinsa Approval; FDA Approval to Seagen’s TUKYSA; NICE Recommends Alnylam’s Amvuttra; FDA Approves Brenzavvy for Type 2 Diabetes; Roche’s Tecentriq to be Filed for Early-stage Liver Cancer; FDA Lifts Hold on Astellas’ Pompe Gene Therapy
- Metastatic Colorectal Cancer Market: Infographics