Nasal Polyposis Market
- Factors contributing to the rise in diagnosed nasal polyposis cases in the 7MM include increased awareness and diagnosis, environmental factors such as air pollution, allergens, and changes in climate patterns that may play a role in triggering inflammation and nasal congestion, which are associated with the development of nasal polyps.
- In the 7MM, the United States accounted for the highest number of diagnosed nasal polyposis cases, totaling approximately 22 million cases in 2023, among these cases, 55% cases were males.
- Among the emerging therapies for nasal polyposis, Fasenra (Benralizumab) is expected to garner the highest market size by 2034 in the 7MM.
- With the expected approval of new therapies during the forecast period (2024–2034), the overall nasal polyposis therapeutic market is projected to experience a significant upsurge at a substantial CAGR.
- Growth of the nasal polyposis market is expected to be mainly driven by the rising prevalence of nasal polyposis, improving diagnostic approaches, an upsurge in the launch of products, and increasing research in pharmaceutical treatment.
DelveInsight’s report titled “Nasal Polyposis Market Insights, Epidemiology, and Market Forecast – 2034” comprehensively analyzes nasal polyposis. The report provides a comprehensive analysis of historical and projected epidemiological data, covering Prevalent Cases of Chronic Rhinosinusitis, Diagnosed Prevalent Cases of Chronic Rhinosinusitis, Diagnosed Prevalent Cases of Nasal Polyposis, and Gender-specific Diagnosed Prevalent Cases of Nasal Polyposis.
The Nasal Polyposis market report provides a comprehensive insight into different facets concerning the patient population, encompassing diagnosis, prescribing trends, physician viewpoints, market accessibility, therapy, and forthcoming market advancements across seven major markets: the United States, EU4 (Germany, France, Italy, and Spain), the UK, and Japan spanning from 2020 to 2034.
The report examines current treatment methodologies and algorithms for nasal polyposis, assessing the overall market potential, identifying business prospects, and addressing pertinent unmet medical requirements.
Nasal Polyposis Overview
Nasal polyposis (NP) is soft, painless, benign inflammatory, and hyperplastic outgrowths of the nasal passages, or sinonasal mucosa. These teardrop-like growths result from chronic inflammation and are associated with asthma, recurring infection, allergies, drug sensitivity, or certain immune disorders.
Nasal polyps are a subgroup of chronic rhinosinusitis. Chronic rhinosinusitis is a condition where the nasal cavity and sinuses are inflamed for more than 4 to 12 weeks. Patients suffering with chronic rhinosinusitis condition can be progressed into chronic rhinosinusitis with nasal polyposis (CRSwNP), and chronic rhinosinusitis without nasal polyposis. Only a portion of chronic rhinosinusitis will develop nasal polyposis.
Chronic rhinosinusitis with nasal polyposis is a potentially debilitating condition in adults that are characterized by inflammation of the nose and paranasal sinuses with the presence of nasal polyps.
People with a history of chronic sinus infections, allergic rhinitis, asthma, and cystic fibrosis, sensitivity to NSAIDs like ibuprofen and aspirin, and Churg–Strauss syndrome are more prone to get nasal polyposis.
Nasal Polyposis Diagnosis and Treatment Algorithm
For the diagnosis of nasal polyps, an ENT specialist or any other physician first look into nasal passages with a special lighted instrument known as a nasoscope. The most preferred techniques are confirming the location, size, and severity of polyp’s nasal endoscopy, and radiological imaging. Further allergy tests and blood tests can be done to determine other disease-related components.
For the treatment of nasal polyposis, Corticosteroids are considered as first-line therapy. Initially, nasal corticosteroids are recommended for treatment. If the patient does not show any progress, then they may be shifted to oral corticosteroids and then to injectable corticosteroids. If the corticosteroids do not show progress in the condition, then surgical intervention can be the next treatment of choice. Biologics can be given to treat severe polyps, to treat post-surgery reoccurrence cases, and to treat patients who cannot undergo surgery. Anti-allergic NSAIDs can be added to the regimen depending on the condition.
Presently, corticosteroids, as well as biologics are available in the market. Apart from this, a few biologics are also present in the emerging pipeline.
Nasal Polyposis Epidemiology
The epidemiology section on nasal polyposis offers an analysis of past and present patient populations, along with projected trends across seven major countries (7MM). Drawing from multiple studies and expert opinions, it aims to elucidate the underlying factors driving current and anticipated trends. Additionally, this segment of the report presents data on the diagnosed patient population, highlighting trends and underlying assumptions.
Key Findings
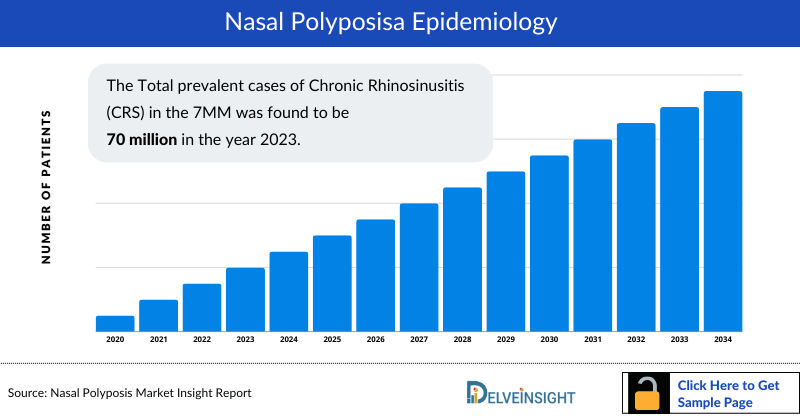
- The Total prevalent cases of Chronic Rhinosinusitis (CRS) in the 7MM was found to be 70 million in the year 2023. These cases are expected to rise significantly by 2034.
- The United States accounted for around 8.5 million diagnosed prevalent cases of chronic rhinosinusitis in 2023.
- In EU4 and the UK, Germany had the highest diagnosed prevalent cases of nasal polyposis with approximately 6 million cases, followed by the UK with around 4 million cases. Projections indicate a substantial change in these cases by the year 2034.
- In 2023, Japan had around 2 million diagnosed prevalent cases of nasal polyposis ranking second-lowest among 7MM. These cases are expected to decrease and by 2034.
- As per DelveInsight analysis, in Japan, the gender-specific diagnosed prevalent cases of nasal polyposis cases were observed to be 110 thousand cases and 90 thousand cases, in men and women respectively in 2023. The gender-specific diagnosed prevalent cases of nasal polyposis are expected to change by 2034.
Nasal Polyposis Drug Chapters
The drug chapter segment of the nasal polyposis report encloses the detailed analysis of nasal Polyposis marketed drugs, mid-phase, and late-stage pipeline drugs. It also helps to understand the nasal polyposis clinical trial details, expressive pharmacological action, agreements and collaborations, approval, and patent details of each included drug, and the latest news and press releases.
Marketed Therapies for Nasal Polyposis
Nucala (Mepolizumab): GlaxoSmithKline
GSK’s Nucala (Mepolizumab) is the first IL-5 therapy approved as an add-on treatment in the US for adults with chronic rhinosinusitis with nasal polyps to target eosinophilic inflammation in adult patients 18 years of age and older with inadequate response to nasal corticosteroids. Nucala got recently approved by US FDA based on positive results from the pivotal Synapse study. After the approval for CRSwNP, Nucala has become the only treatment approved in the US for use in four eosinophil-driven diseases. Nucala has contributed to giving one of the broadest portfolios to GSK in respiratory medicines.
Xhance (Fluticasone Propionate): Optinose US Inc.
Xhance (fluticasone propionate) is an intranasal spray (INS) corticosteroid indicated for the treatment of nasal polyps in patients 18 years of age or older (FDA, 2017a). Xhance is a combination of Exhalation Delivery Systems (EDS) with a liquid formulation of fluticasone propionate, a well-characterized, second-generation corticosteroid. Xhance is designed to deliver medication into the high and deep regions of the nasal passages where both nasal polyps and inflamed and swollen membranes can obstruct normal sinus ventilation and drainage.
Note: Detailed Marketed therapies assessment and list of products to be continued in the report...
Emerging Therapies for Nasal Polyposis
The potential drugs that are expected to launch in the forecasted period include Fasenra (benralizumab), Tezepelumab, and others.
Fasenra (benralizumab): AstraZeneca
Fasenra (benralizumab), being developed by AstraZeneca, is a monoclonal antibody that binds directly to IL-5 receptor alpha on eosinophils and attracts natural killer cells to induce rapid and near-complete depletion of eosinophils via apoptosis (programmed cell death). Fasenra targets seven diseases beyond respiratory disease.
Interleukin-5 (IL-5) induces an eosinophil-mediated inflammatory response by binding to the IL-5 receptor (IL-5R) expressed in eosinophils, basophils and some mast cells. Benralizumab, unlike IL-5 low-affinity binding, binds with high affinity to the domain I of the α-chain of IL-5R and blocks its signaling and the proliferation of IL-5-dependent cell lines. On the other hand, Benralizumab is an afucosylated antibody in the CH2 region, giving it a high affinity for the FcγRIIIa on natural killer cells, macrophages, and neutrophils; this binding triggers a magnified apoptosis response in eosinophils via antibody-dependent cell-mediated cytotoxicity.
Tezepelumab: AstraZeneca/Amgen
AstraZeneca is developing Tezepelumab as a potential human monoclonal antibody that inhibits the action of thymic stromal lymphopoietin (TSLP). TSLP is an epithelial-derived cytokine produced in response to airborne stimuli, such as allergens, viruses, and diesel exhaust. It plays a key role in the initiation and persistence of airway inflammation by activating multiple downstream inflammatory pathways. TSLP expression is elevated in the airways of patients with asthma compared with healthy individuals and in nasal polyp tissue from patients with CRSwNP compared with healthy sinus tissue or that from patients with CRS without NP. The presence of TSLP correlates with the number of eosinophils in nasal polyp tissue, and the activity of TSLP in polyp tissue is believed to be sufficient to trigger an inflammatory response. In patients with asthma, the level of TSLP expression in airway tissue has been shown to correlate with airway obstruction and disease severity. Tezepelumab is a human monoclonal antibody (immunoglobulin [Ig] G2λ) that binds specifically to TSLP, preventing its interaction with its heterodimeric receptor.
Tezepelumab is in Phase III clinical trial for nasal polyps and other indications. The positive response for CRSwNP Tezepelumab was explored from the clinical trials; specifically, Pathway and Navigator, dedicated for the Asthma indication.
Note: Detailed emerging therapies assessment and list of products to be continued in the report....
Nasal Polyposis Market Outlook
The standard treatment for nasal polyposis includes a combination of pharmacological and surgical approaches depending on individual case assessment. In general, patients are treated medically in the primary care setting before considering surgical procedures. Treatment aims to eliminate or significantly reduce the size of the nasal polyps resulting in relief of nasal obstruction, improvement in sinus drainage, restoration of olfaction and taste. Treatment of any accompanying rhinitis symptoms may also be required. With both treatments, recurrences are common, particularly in patients with asthma who are twice as likely to develop recurrence compared with nonasthmatics.
Presently, for the treatment of nasal polyposis corticosteroids (intranasal and systemic) and biologics (Dupixent, Xolair, and Nucala) can be used. Corticosteroid implants such as Propel and Sinuva are also available to improve the outcomes of surgery and to be used in recurrent nasal polyposis, respectively. In addition anti-histamines, antibiotics, and NSAIDs can be given depending on the patient's need. If the patient does not show improvement by therapeutics agents then surgery can be also performed to remove the polyps.
Intranasal glucocorticoids (budesonide, fluticasone, and mometasone) may decrease rhinorrhea, reduce polyp size, and restore the sense of smell, entirely or partially.
If topical treatment fails to produce the desired relief in 4–6 weeks, as is often the case, patients may need a short course of systemic steroids. Using oral glucocorticoids for 2–3 weeks may alleviate symptoms and reduce polyp size, improving quality of life. Unfortunately, the relief may last only 3–6 months, at which point patients may need another course of systemic glucocorticoids.
Some biologics are also preferred treatment options in the NP patient group. Interleukins 4 and 13 may aggravate nasal polyps, and inhibition with dupilumab (Dupixent) has been shown to decrease polyp burden and improve symptoms.
Note: Detailed market outlook to be continued in the report....
Nasal Polyposis Market Segmentation
DelveInsight’s ‘Nasal Polyposis – Market Insights, Epidemiology, and Market Forecast – 2034’ report provides a detailed outlook of the current and future nasal polyposis market, segmented within countries and by therapies. Further, the market of each region is then segmented by each therapy to provide a detailed view of the current and future market share of all therapies.
Nasal Polyposis Market Size by Countries
The total nasal polyposis market size is analyzed for individual countries (the United States Market, EU4 (Germany, France, Italy, and Spain) and the UK market, and Japan). The United States accounted for a larger portion of the 7MM market for nasal polyposis in 2023 due to the high prevalence of the condition and high treatment cost. This dominance is predicted to continue with the potential early entry of new products.
Country-wise Market Size Distribution of Nasal Polyposis
Nasal Polyposis Market Size by Therapies
From the last 5 years, biologics have started to come into the market for both the pool (not responding to initial treatment and recurrent cases from surgery). Now, only the severe nasal polyps patients are preferred to take biologics. As with the launch of more biologics in the upcoming years, the ratio of patients opting for surgery might reduce due to the excellent efficacy of biologics and also less possibility of recurrence of the disease.
The approved therapies in the US for the treatment of nasal polyposis include NUCALA (mepolizumab) by GlaxoSmithKline, XHANCE (fluticasone propionate) by Optinose US Inc., and DUPIXENT by Sanofi/Regeneron Pharmaceuticals among others.
It is believed that the nasal polyposis treatment market could change by 2034, in the US with the launch of novel therapies with better and broader efficacy, treatment duration, better dosages, and enhanced convenience.
Key Findings
- The market size of nasal polyposis in 7MM was nearly USD 1,910 million in 2023, which is further expected to increase by 2034 at a significant Compound Annual Growth Rate (CAGR) for the study period (2020–2034).
- The United States accounts for the largest market size for nasal polyposis accounting for around 72% of the total 7MM market size.
- Upcoming therapies such as Fasenra (benralizumab) and Tezepelumab have the potential to create a significant positive shift in the nasal polyposis market size.
- Among the European countries, Germany had the highest market size with USD 154 million in 2023.
- The expected Launch of potential therapies may increase market size in the coming years, assisted by an increase in the diagnosed prevalent population of nasal polyposis.
Note: Detailed market segment assessment will be provided in the final report....
Nasal Polyposis Drugs Uptake
This section focuses on the sales uptake of potential nasal polyposis drugs that have recently launched or are anticipated to be launched in the nasal polyposis market between 2020 and 2034. It estimates the market penetration of the nasal polyposis drugs for a given country, examining their impact within and across classes and segments. It also touches upon the financial and regulatory decisions contributing to the drug’s probability of success (PoS) in the nasal polyposis market.
For example, for Fasenra (Benralizumab), we expect the drug uptake to be medium with a probability-adjusted peak share of around 5.2%, and years to the peak is expected to be 6 years from the year of launch in the US.
Note: Detailed assessment of drug uptake will be provided in the full report on Nasal Polyposis....
Nasal Polyposis Market Access and Reimbursement
DelveInsight’s ‘Nasal Polyposis – Market Insights, Epidemiology, and Market Forecast – 2034’ report provides a descriptive overview of the market access and reimbursement scenario of nasal polyposis.
This section includes a detailed analysis of the country-wise healthcare system for each therapy, enlightening the market access, reimbursement policies, and health technology assessments.
KOL Views
To keep up with current nasal polyposis market trends and fill gaps in secondary findings, we interview KOLs and SMEs working in the nasal polyposis domain. Their opinion helps understand and validate current and emerging therapies and treatment patterns or nasal polyposis market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the nasal polyposis unmet needs.
Nasal Polyposis: KOL Insights
DelveInsight’s analysts connected with 50+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. These KOLs were from organizations, institutes, and hospitals, such as Wright State University Boonshoft School of Medicine, Fairborn, US, University of California San Diego, San Diego, California, US, and University of California-San Diego, San Diego, CA, US, University of Siena, Italy, University of Siena, Italy, University of Fukui, Fukui, Japan and others.
“After CRS is diagnosed, surgery is only advised when optimal pharmacological management shows no sign of improvement in symptoms even after 3 months. Even studies have showed that primary surgery before medical treatment show no clear benefit over primary pharmacological treatment.”
Note: Detailed assessment of KOL Views will be provided in the full report of Nasal Polyposis....
Competitive Intelligence Analysis
We perform Competitive and Market Intelligence analysis of the nasal polyposis market using various Competitive Intelligence tools, including SWOT analysis, conjoint analysis, Market entry strategies, etc. The inclusion of the analysis entirely depends upon the data availability.
The emerging nasal polyposis therapies are analyzed based on various attributes such as safety and efficacy in randomized clinical trials, order of entry and other market dynamics, and the unmet need they fulfill in the nasal polyposis market.
Note: Detailed assessment of SWOT analysis and conjoint analysis will be provided in the full report on Nasal Polyposis....
Nasal Polyposis Pipeline Development Activities
The report provides insights into therapeutic candidates in Phase II and III stages. It also analyzes nasal polyposis Companies involved in developing targeted therapeutics.
Pipeline Development Activities
The report covers information on collaborations, acquisition and merger, licensing, patent details, and other information for emerging nasal polyposis therapies.
Scope of the Report
- The report covers a segment of key events, an executive summary, descriptive overview of nasal polyposis, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies
- Comprehensive insight has been provided into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, disease progression along with treatment guidelines
- Additionally, an all-inclusive account of both the current and emerging therapies along with the elaborative profiles of late-stage and prominent therapies will have an impact on the current treatment landscape
- A detailed review of the nasal polyposis market; historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach
- The report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preference that help in shaping and driving the 7MM nasal polyposis market
Nasal Polyposis Report Insights
- Patient Population
- Therapeutic Approaches
- Nasal Polyposis Pipeline Analysis
- Nasal Polyposis Market Size and Trends
- Existing and future Market Opportunity
Nasal Polyposis Report Key Strengths
- 11 Years Forecast
- 7MM Coverage
- Nasal Polyposis Epidemiology Segmentation
- Key Cross Competition
- Attribute analysis
- Drugs Uptake and Key Market Forecast Assumptions
Nasal Polyposis Report Assessment
- Current Treatment Practices
- Unmet Needs
- Pipeline Product Profiles
- Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
Key Questions
Market Insights:
- What was the nasal polyposis total market size, the market size by therapies, and market share (%) distribution in 2020, and how it would all look in 2034? What are the contributing factors for this growth?
- What are the unmet needs are associated with the current treatment market of nasal polyposis?
- How is Fasenra (benralizumab) going to contribute to the market of nasal polyposis after approval?
- Which drug is going to be the largest contributor in 2034?
- What are the pricing variations among different geographies for approved and off-label therapies?
- How would the market drivers, barriers, and future opportunities affect the market dynamics and subsequent analysis of the associated trends?
Epidemiology Insights:
- What are the disease risk, burden, and unmet needs of nasal polyposis? What will be the growth opportunities across the 7MM concerning the patient population of nasal polyposis?
- What is the historical and forecasted nasal polyposis patient pool in the United States, EU4 (Germany, France, Italy, and Spain) the United Kingdom, and Japan?
- Why do only limited patients appear for diagnosis? Why is the current year diagnosis rate not high?
- What factors are affecting the diagnosis and treatment of the indication?
Current Treatment Scenario, Marketed Drugs, and Emerging Therapies:
- What are the current options for the treatment of nasal polyposis? What are the current treatment guidelines for the treatment of nasal polyposis in the US and Europe?
- How many companies are developing therapies for the treatment of nasal polyposis?
- How many emerging therapies are in the mid-stage and late stage of development for the treatment of nasal polyposis?
- What are the recent novel therapies, targets, mechanisms of action, and technologies developed to overcome the limitation of existing therapies?
- What are the key designations that have been granted for the emerging therapies for nasal polyposis?
- Patient acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the country-specific accessibility issues of expensive, recently approved therapies? Focus on reimbursement policies.
- What are the 7MM historical and forecasted market of nasal polyposis?
Reasons to buy
- The report will help in developing business strategies by understanding the latest trends and changing treatment dynamics driving the nasal polyposis Market.
- Insights on patient burden/disease prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years
- To understand the existing market opportunity in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identification of strong upcoming players in the market will help in devising strategies that will help in getting ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the Conjoint analysis section to provide visibility around leading classes
- Highlights of Access and Reimbursement policies of approved therapies, barriers to accessibility of off-label expensive therapies, and patient assistance programs
- To understand the perspective of Key Opinion Leaders’ around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in future.
- Detailed insights on the unmet need of the existing market so that the upcoming players can strengthen their development and launch strategy
Frequently Asked Questions
1. What are the treatment goals for Nasal Polyposis?
The primary treatment goals for nasal polyposis involve relieving symptoms, reducing inflammation, improving nasal airflow, restoring the sense of smell, preventing recurrence, preserving sinus function, and enhancing overall quality of life for patients.
2. What are the challenges in managing Nasal Polyposis?
Managing nasal polyposis presents several challenges. One key challenge is the chronic and recurrent nature of the condition, which often necessitates long-term treatment and monitoring to control symptoms and prevent polyp regrowth.
3. What are the key factors driving the growth of the Nasal Polyposis market?
The Nasal Polyposis market is propelled by factors like increasing prevalence, medical advancements, rising awareness, and the introduction of novel therapies by key pharmaceutical players. These elements fuel demand for innovative treatments, addressing unmet medical needs and driving market expansion.
4. How will the Nasal Polyposis Market and Epidemiology Forecast Report benefit the clients?
The report will provide comprehensive insights into the current nasal polyposis market landscape, emerging therapies, competitive dynamics, regulatory requirements, and market access considerations, enabling informed decision-making, strategic planning, and optimization of business strategies to capitalize on market opportunities and drive growth.