Netherton Syndrome Market Summary
- The Netherton syndrome in the 7MM was valued at approximately USD 25 million in 2024. Over the forecast period from 2025 to 2034, this market is projected to grow at a CAGR of 7% in leading countries like the US, EU4, the UK, and Japan.
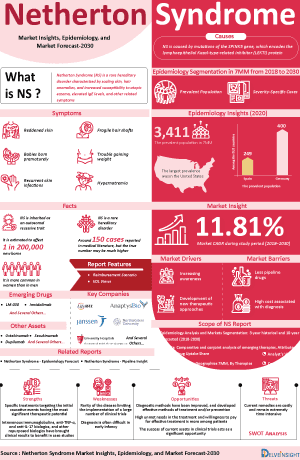
Netherton Syndrome Market and Epidemiology Analysis
- According to DelveInsight, there were around 3,500 diagnosed prevalent cases of Netherton syndrome across the 7MM in 2024.
- The Netherton syndrome market is expected to maintain a consistent compound annual growth rate (CAGR) throughout the forecast period (2025–2034). This revenue growth is largely driven by the development of new therapies, improvements in diagnostic methods, increased awareness of the condition, and a rising number of diagnosed cases. Azitra with ATR-12, Quoin Pharmaceuticals with QRX003, Boehringer Ingelheim with SPEVIGO, and Daiichi Sankyo with DS-2325 are playing a major role in building the pipeline.
- Netherton syndrome currently has no specific cure or approved therapies for its management, resulting in a substantial unmet need for patients. While treatments such as emollients, and keratolytics may offer symptomatic relief, they do not address the underlying condition, highlighting the need for more targeted and effective therapeutic options.
- In April 2025, Quoin Pharmaceuticals reported positive pediatric study results for QRX003 in Netherton syndrome, followed in June 2025 by the US Food and Drug Administration (FDA) granting Rare Pediatric Disease Designation (RPDD), enabling potential Priority Review Voucher eligibility upon approval.
DelveInsight’s “Netherton Syndrome Market Insights, Epidemiology, and Market Forecast – 2034” report delivers an in-depth understanding of Netherton syndrome, historical and forecasted epidemiology, as well as the Netherton syndrome market trends in the United States, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
The Netherton syndrome market report provides current treatment practices, emerging drugs, market share of individual therapies, and current and forecasted 7MM Netherton syndrome market size from 2020 to 2034. The report also covers Netherton syndrome treatment practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s potential.
|
Study Period |
2020–2034 |
|
Forecast Period |
2025–2034 |
|
Geographies Covered |
US, EU4 (Germany, France, Italy, and Spain), the UK, and Japan |
|
Netherton Syndrome Epidemiology |
|
|
Netherton Syndrome Market |
|
|
Market Analysis |
|
|
Netherton Syndrome Market Players |
|
|
Future Opportunity |
The outlook for Netherton syndrome treatment is encouraging, with several companies—such as Quoin Pharmaceuticals, Daiichi Sankyo, and Azitra—actively developing therapies that have the potential to address the current gap in targeted treatment options. These ongoing efforts reflect a growing commitment to advancing care for this rare condition and improving outcomes for affected patients. |
Netherton Syndrome: Understanding and Treatment Algorithm
Netherton Syndrome Overview
Netherton Syndrome is a rare and severe autosomal recessive genetic disorder classified under ichthyosis. It is an inherited skin condition that typically appears at birth or within the first few weeks of life, characterized by red, inflamed skin covered with fine, dry scales. Although lifelong, the severity of the condition can vary significantly between individuals. Also known as Comèl-Netherton Syndrome, it is clinically defined by a triad of features: Ichthyosis Linearis Circumflexa (ILC), trichorrhexis invaginata (commonly referred to as bamboo hair), and atopic manifestations, along with multisystemic complications.
Bamboo hair reflects abnormalities in the hair shaft, while atopic diathesis is associated with frequent allergic conditions such as asthma and eczema. The ichthyosiform erythroderma leads to widespread red and scaly skin. The syndrome typically manifests at or shortly after birth, with symptoms including generalized redness, itching, and scaling. Affected individuals may also suffer from recurrent infections, delayed growth, and a significantly impacted quality of life.
Netherton Syndrome Diagnosis
Diagnosing Netherton syndrome in early infancy can be challenging, as its clinical features—such as erythroderma and failure to thrive—are also common in other conditions, including various immune deficiency disorders. Diagnosis typically involves a combination of clinical assessment, evaluation of family history, and skin biopsy. To confirm the presence of SPINK5 gene mutations, molecular genetic testing is often employed.
While identifying a germline SPINK5 mutation through DNA sequencing can strongly support the diagnosis, the high cost of this method may limit its widespread use. Additionally, several conditions must be considered during differential diagnosis, including peeling skin syndrome, omenn syndrome, other primary immunodeficiency syndromes, hyper IgE syndromes, severe atopic dermatitis and contact dermatitis, multiple allergies, and metabolic disorders such as SAM (severe atopic and metabolic) syndrome.
Further details related to country-based variations are provided in the report…
Netherton Syndrome Treatment
The management of Netherton syndrome involves a multidisciplinary approach focused on symptom relief rather than addressing the underlying genetic cause. Currently, there are no approved treatments specifically for this condition. Symptomatic therapies include the use of emollients, keratolytics, and moisturizers to maintain skin hydration, as well as topical corticosteroids to alleviate inflammation, itching, and redness. Additionally, topical vitamin D analogs like calcipotriol are used to reduce scaling and inflammation, while calcineurin inhibitors such as pimecrolimus and tacrolimus help regulate immune responses and control skin inflammation.
Other treatments may include retinoids, immunosuppressants (both topical and systemic), and various anti-inflammatory agents. Biologics are sometimes prescribed off-label; however, their effectiveness in Netherton syndrome has yet to be fully established. It's important to note that many of these therapies, especially with prolonged use, carry safety concerns, including risks of skin thinning, irritation, and recurrent infections.
Netherton Syndrome Epidemiology
As the market is derived using a patient-based model, the Netherton syndrome epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by, total diagnosed prevalent cases of Netherton syndrome, gender-specific diagnosed prevalent cases of Netherton syndrome, and treated cases of Netherton syndrome in the 7MM covering the United States, EU4 countries (Germany, France, Italy, and Spain), the United Kingdom, and Japan from 2020 to 2034.
- In EU4 and the UK, females constituted a higher proportion of affected individuals, accounting for approximately 58% of cases, compared to 42% in males, highlighting a notable gender disparity in disease burden.
- In 2024, Germany recorded the highest number of gender-specific diagnosed prevalent cases among EU4 and the UK, with approximately 245 cases in females and 175 cases in males.
- According to DelveInsight analysis, in 2024, there were nearly 300 diagnosed prevalent cases of Netherton syndrome reported in Italy.
- In 2024, Japan reported approximately 123 identified cases of Netherton syndrome, highlighting the condition’s rarity and limited patient population.
Netherton Syndrome Recent Developments
- In September 2025, ResVita Bio announced a successful Pre-IND meeting with the FDA for RVB-003, its lead investigational therapy for Netherton Syndrome—a rare, life-threatening genetic skin disease caused by SPINK5 gene mutations. The condition involves severe skin inflammation and barrier defects, and currently has no FDA-approved treatments.
Netherton Syndrome Drug Chapters
The drug chapter segment of the Netherton syndrome report encloses a detailed analysis of Netherton syndrome early to late–stage (Phase I and Phase II/II) pipeline drugs. It also helps understand the Netherton syndrome clinical trial details, expressive pharmacological action, agreements and collaborations and approval, advantages and disadvantages of each included drug, and the latest news and press releases.
Currently, there are no approved targeted therapies specifically for the treatment of Netherton syndrome. Management primarily relies on symptomatic treatments such as emollients, corticosteroids, and keratolytics, which help to hydrate the skin, alleviate lesions, and provide temporary relief. Given the absence of disease-specific approved options, there remains a substantial unmet need in this space. The development and eventual approval of targeted therapies could address this gap, improve patient outcomes, and foster healthy competition in the market, ultimately contributing to increased therapeutic innovation and market growth.
With ongoing research and continued dedication, the future holds hope for even more effective treatments and, ultimately, a cure for this challenging condition. According to DelveInsight, the Netherton syndrome market in the 7MM is expected to change significantly during the study period 2020–2034.
Netherton Syndrome Emerging Drugs
The Netherton syndrome market is anticipated to undergo gradual transformation, primarily due to the limited pipeline of emerging therapies. However, key players such as Azitra with ATR-12, Quoin Pharmaceuticals with QRX003, Boehringer Ingelheim with SPEVIGO (spesolimab), and Daiichi Sankyo with DS-2325 have shown strong interest in this rare condition and are actively engaged in developing promising treatment options.
ATR-12: Azitra
ATR-12, currently in Phase I development by Azitra for Netherton Syndrome, utilizes a bioengineered strain of Staphylococcus epidermidis (SE351) to topically deliver therapeutic levels of the lymphoepithelial Kazal-type-related inhibitor (LEKTI) protein—an essential serine protease inhibitor lacking in affected patients. By restoring LEKTI directly at the skin barrier, ATR-12 aims to address the underlying protein deficiency driving the disease. Notably, ATR-12 has also received RPDD from the US FDA, underscoring its potential as a promising therapeutic approach for this underserved pediatric population
In August 2025, Azitra reported initial safety findings and 50% enrollment in its Phase Ib trial of ATR-12 for Netherton syndrome, showing a favorable safety profile, with topline results anticipated in first quarter of 2026 to guide further clinical development.
In May 2024, Azitra shared encouraging preclinical results for ATR-12 and unveiled its clinical development strategy for Netherton syndrome during a presentation at the American Society of Gene and Cell Therapy (ASGCT) Annual Meeting, marking a key step forward in advancing this investigational therapy.
DS-2325: Daiichi Sankyo
DS-2325 is an investigational drug developed by Daiichi Sankyo for Netherton syndrome, a severe genetic skin disorder characterized by widespread scaling. DS-2325 works by selectively inhibiting KLK5, a serine protease that becomes hyperactive in Netherton syndrome due to deficient LEKTI protein levels. This hyperactivity leads to uncontrolled skin shedding and inflammation. By blocking KLK5, DS-2325 aims to restore protease balance, improve epidermal barrier function, and reduce symptoms like scaling and redness
DS-2325 has received multiple regulatory designations from the US FDA, including Orphan Drug Designation (ODD), Fast Track Designation (FTD), and RPDD. These designations are expected to facilitate a smoother and potentially accelerated approval process, ultimately helping to make the therapy more accessible to patients in need.
The current trial has been terminated, though the company has not formally withdrawn the program at this stage.
SPEVIGO: Boehringer Ingelheim
SPEVIGO (spesolimab) is a humanized monoclonal antibody (mAb) that targets the interleukin-36 receptor (IL-36R). It is currently under clinical development for Netherton syndrome—a rare, inherited skin disorder characterized by chronic inflammation, scaling, and hair abnormalities.
SPEVIGO is currently in Phase II/III clinical trials as a potential treatment for Netherton syndrome and has also received ODD from the US FDA for this indication.
|
Drug |
MoA |
RoA |
Company |
Logo |
Phase |
|
ATR-12 |
Serine peptidase inhibitor kazal-type 5 replacement |
Topical |
Azitra |
|
I |
|
DS-2325 |
KLK5 Inhibitor |
IV and SC |
Daiichi Sankyo |
|
I/II |
|
SPEVIGO |
IL- 36 receptor antagonist |
IV |
Boehringer Ingelheim |
|
II/III |
Note: Further emerging therapies and their detailed assessment will be provided in the final report.
Netherton Syndrome Drug Class Insights
Currently, Netherton syndrome is primarily managed with off-label therapies such as emollients and keratolytics, which help soften the skin and alleviate lesions. Additional treatment approaches may include retinoids, immunosuppressants (both topical and systemic), and various anti-inflammatory agents. While biologics are occasionally used off-label, their clinical efficacy in Netherton syndrome remains to be fully validated. It is important to consider that many of these treatment options, particularly with long-term use, may be associated with safety concerns such as skin thinning, irritation, and an increased risk of infections.
- Emerging therapies in development include ATR-12 by Azitra, DS-2325 by Daiichi Sankyo, and SPEVIGO (spesolimab) by Boehringer Ingelheim.
- ATR-12 developed by Azitra is currently under Phase Ib which act by serine peptidase inhibition kazal-type 5 replacement.
- DS-2325, developed by Daiichi Sankyo, it is also a KLK5 inhibitor, it is currently in Phase I/II clinical trials for the treatment of Netherton syndrome.
- SPEVIGO (spesolimab), developed by Boehringer Ingelheim acts on IL- 36 receptor antagonists, it is currently in Phase II/III for the treatment of Netherton syndrome.
Continued in report…
Netherton Syndrome Market Outlook
There are currently no approved therapies specifically indicated for Netherton syndrome, and management largely relies on off-label and symptomatic treatments. Agents such as retinoids, immunosuppressants (topical and systemic), and anti-inflammatory medications are commonly employed, though their effectiveness in this rare condition remains incompletely validated. Biologics are also occasionally prescribed off-label, but conclusive evidence supporting their benefit is still limited. Symptomatic care typically includes emollients, keratolytics, and moisturizers to support skin barrier function and hydration. Topical corticosteroids are used to manage inflammation, pruritus, and erythema, while topical vitamin D analogs like calcipotriol help reduce scaling. Calcineurin inhibitors such as pimecrolimus and tacrolimus offer an alternative for modulating immune activity and controlling skin inflammation, although their long-term safety and efficacy profiles continue to be closely evaluated.
There is a significant need for new treatments for Netherton syndrome, as current therapies limited to off labels are use of generic. Several promising drugs are currently in the pipeline, including SPEVIGO, ATR-12, DS-2325, and QRX003, among others.
- The total Netherton syndrome market size was valued at USD 25 million in 2024 across the 7MM, and this market is projected to grow moving forward throughout the forecast period (2025-2034).
- In 2024, the US accounted for an estimated market size of approximately USD 15 million for Netherton syndrome, representing nearly 65% of the total revenue generated across the 7MM. This highlights the dominant share of the US market in the overall commercial landscape for the condition.
- In 2024, the Netherton syndrome market size in EU4 and the UK were around USD 8 million, accounting for 32% of the total market. This figure is expected to grow significantly with the introduction of emerging therapies.
- In 2024, Germany's therapy-wise market for Netherton syndrome was valued at approximately USD 2 million. This included a range of treatment options such as topical calcineurin inhibitors (TCIs), other therapies like retinoids, IVIG, biologics, corticosteroids, as well as emerging treatments such as QRX003 and SPEVIGO (spesolimab), reflecting a diverse and evolving therapeutic landscape.
- In 2024, the treatment market for Netherton syndrome in Japan was valued less than one million. This figure is anticipated to grow in the coming years, indicating potential for increased revenue as therapeutic options evolve and awareness of the condition improves.
Netherton Syndrome Drugs Uptake
This section focuses on the uptake rate of potential drugs expected to be launched in the market during 2020–2034.
Further detailed analysis of emerging therapies drug uptake in the report…
Netherton Syndrome Pipeline Development Activities
The report provides insights into different therapeutic candidates in Phase III, Phase II, and Phase I. It also analyzes key players involved in developing targeted therapeutics.
Pipeline development activities
The report covers information on collaborations, acquisitions and mergers, licensing, and patent details for emerging therapies for Netherton syndrome.
KOL Views
To keep up with current market trends, we take KOLs and SMEs’ opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts contacted for insights on Netherton syndrome evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake, along with challenges related to accessibility, including Medical/scientific writers, Medical Professionals, Professors, Directors, and Others.
DelveInsight’s analysts connected with 50+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. Centers like Parkview Health, US, Meyer Children's University Hospital, Florence, Italy, Giovan Battista Mattei Research Institute, Stenico, Italy, and Department of Dermatology and Kurume University School of Medicine, Japan, among others, were contacted. Their opinion helps understand and validate current and emerging therapy treatment patterns or Netherton syndrome market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
Physician’s View
As per the KOLs from the US, “Current treatment options for Netherton syndrome are primarily limited to offering modest symptomatic relief. Although moisturizers are commonly used, those containing lanolin or petrolatum may exacerbate skin damage in patients with fragile skin due to friction or shear stress during application or removal. Similarly, standard treatments like calcineurin inhibitors may result in dangerously elevated systemic absorption because of the impaired skin barrier, posing additional safety concerns.”
As per the KOLs from Germany, “Netherton Syndrome is caused by mutations in the SPINK5 gene, which encodes the LEKT, a protein found in hair follicles and the granular layer of the skin’s epidermis. A deficiency or malfunction of LEKTI leads to increased serine protease activity, which disrupts the skin barrier by causing early detachment of the stratum corneum, ultimately impairing skin function.”
As per the KOLs from Japan, “Patients with Netherton syndrome face a notably increased risk of developing allergies to food and environmental triggers compared to individuals with other skin disorder phenotypes. In contrast, those with keratitis-ichthyosis-deafness (KID) syndrome are significantly more prone to experiencing skin infections than patients with other related conditions.”
Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT and Attribute Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Attribute analysis analyzes multiple emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
To analyze the effectiveness of these therapies, have calculated their attributed analysis by giving them scores based on their ability to improve the symptoms of the indication.
Further, the therapies’ safety is evaluated wherein the adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials, which directly affects the safety of the molecule in the upcoming trials. It sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the route of administration, order of entry and designation, probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Scope of the Report
- The report covers a segment of key events, an executive summary, and a descriptive overview of Netherton syndrome explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight into the epidemiology segments and forecasts, the future growth potential of prevalence rate, disease progression, and treatment guidelines have been provided.
- Additionally, an all-inclusive account of the current and emerging therapies and the elaborative profiles of late-stage and prominent therapies will impact the current treatment landscape.
- A detailed review of the Netherton syndrome, market, and historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The report provides an edge while developing business strategies by understanding trends through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help shape and drive the 7MM Netherton syndrome.
Netherton Syndrome Report insights
- Netherton Syndrome Patient Population
- Netherton Syndrome Therapeutic Approaches
- Netherton Syndrome Pipeline Analysis
- Netherton Syndrome Market Size and Trends
- Existing and Future Netherton Syndrome Market Opportunity
Netherton Syndrome Report Key Strengths
- 10 years Forecast
- The 7MM Coverage
- Netherton Syndrome Epidemiology Segmentation
- Key Cross Competition
- Attribute Analysis
- Drugs Uptake and Key Market Forecast Assumptions
Netherton Syndrome Report Assessment
- Current Netherton Syndrome Treatment Practices
- Netherton Syndrome Treatment Unmet Needs
- Netherton Syndrome Pipeline Product Profiles
- Netherton Syndrome Market Attractiveness
- Qualitative Analysis (SWOT and Attribute Analysis)
Key Questions
Market Insights
- What are the key findings of Netherton syndrome epidemiology across the 7MM, and which country will have the highest number of patients during the study period (2020–2034)?
- At what CAGR is the Netherton syndrome market and its epidemiology is expected to grow in the 7MM during the forecast period (2025–2034)?
- What would be the forecasted patient pool of Netherton syndrome in the 7MM covering the United States, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan?
- Which drug is going to be the largest contributor by 2034?
- How would future opportunities affect the market dynamics and subsequent analysis of the associated trends?
Epidemiology Insights
- What are the disease risks, burdens, and unmet needs of Netherton syndrome? What will be the growth opportunities across the 7MM with respect to the patient population pertaining to Netherton syndrome?
- What is the historical and forecasted Netherton syndrome patient pool in the United States, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan?
- Out of the countries mentioned above, which country would have the highest prevalent cases of Netherton syndrome population during the forecast period (2025–2034)?
- What factors are contributing to the growth of Netherton syndrome cases?
Current Treatment Scenario, Marketed Drugs, and Emerging Therapies
- What are the current options for the treatment of Netherton syndrome?
- What are the current clinical and treatment guidelines for treating Netherton syndrome?
- How many companies are developing therapies for the treatment of Netherton syndrome?
- How many emerging therapies are in the mid-stage and late stage of development for treating Netherton syndrome?
- What are the recent novel therapies, targets, mechanisms of action, and technologies developed to overcome the limitations of existing therapies?
- What is the cost burden of current treatment on the patient?
- Patient acceptability in terms of preferred treatment options as per real-world scenarios?
- What is the 7MM historical and forecasted market of Netherton syndrome?
Reasons to Buy
- The report will help in developing business strategies by understanding the latest trends and changing treatment dynamics driving the Netherton syndrome market.
- Insights on patient burden/disease prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- To understand the existing market opportunity in varying geographies and the growth potential over the coming years.
- The distribution of historical and current patient share is based on real-world prescription data in the US, EU4 (Germany, France, Italy, and Spain), the UK, and Japan.
- Identifying upcoming solid players in the market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of class-wise potential emerging therapies under the attribute analysis section to provide visibility around leading classes.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing market so that the upcoming players can strengthen their development and launch strategy.