oncolytic virus cancer therapy competitive landscape
DelveInsight’s, “Oncolytic Virus Cancer Therapy - Competitive landscape, 2026,” report provides comprehensive insights about 100+ companies and 110+ drugs in Oncolytic Virus Cancer Therapy Competitive landscape. It covers the therapeutics assessment by product type, stage, route of administration, and molecule type. It further highlights the inactive pipeline products in this space.
Geography Covered
- Global coverage
Oncolytic Virus: Understanding
Oncolytic Virus: Overview
Oncolytic viruses (OVs) are a promising class of cancer therapeutics distinguished by their ability to selectively replicate in tumor cells, deliver complex transgene payloads, induce immunogenic cell death, and stimulate antitumor immunity, while maintaining a safety profile largely distinct from conventional therapies. Although the concept of using viruses to treat cancer dates back nearly a century, modern progress accelerated in the 1990s with the advent of genetically engineered OVs and has since led to substantial clinical development and numerous trials. Advances in viral biology, tumor immunology, and molecular genetics have clarified how OVs can exploit natural or engineered tumor tropism to infect and lyse malignant cells and remodel the immunosuppressive tumor microenvironment, including counteracting immune evasion mechanisms such as aberrant activation of immune checkpoints like CTLA-4 and PD-1/PD-L1. By converting immunologically “cold” tumors into inflamed, immune-responsive sites, OVs enhance immune-mediated tumor clearance, particularly when combined with other cancer therapies, a strategy now being evaluated in over 100 clinical trials. Clinically, OVs can be administered via multiple routes, including intratumoral, intravenous, and regional infusions, and are generally well tolerated, with transient flu-like symptoms being the most common adverse effects.
Viruses are obligate intracellular particles composed of a nucleic acid genome enclosed within a protein capsid and, in some cases, a surrounding lipid envelope, with their genetic material dictating viral structure and function. Advances in virology and genetic engineering have enabled the rational modification of viruses for therapeutic applications, including vaccines, gene delivery vectors, and cell engineering tools. Often described as nature’s nanoparticles (20–500 nm), oncolytic viruses (OVs) are uniquely capable of selectively infecting and destroying cancer cells while sparing normal tissue by exploiting tumor-specific receptors or aberrant gene expression, with viral genes mediating cytotoxicity and the capsid serving as an efficient nucleic acid delivery vehicle.
Unlike conventional chemotherapy, OVs can be precisely engineered and optimized based on preclinical and clinical insights. Upon entry into the host, viral components act as pathogen-associated molecular patterns recognized by dendritic cells through toll-like receptors, triggering the release of type I interferons, TNF-a, IL-2, and other cytokines that recruit immune cells and sustain inflammation. OVs may be classified as DNA or RNA viruses, each with distinct advantages and limitations: DNA viruses exhibit high genetic stability due to faithful polymerases but show weaker immunogenicity and carry a potential risk of genomic integration, whereas RNA viruses are smaller, more immunostimulatory, disseminate more efficiently, and are particularly effective against central nervous system tumors due to their ability to spread systemically and cross the blood–brain barrier.
Oncolytic viruses (OVs) eliminate cancer through a dual mechanism of selective tumor cell lysis and potent immune activation. They preferentially infect and replicate in cancer cells by exploiting tumor-specific receptors, dysregulated signaling pathways, altered metabolism, and impaired antiviral defenses, leading to organelle dysfunction, oxidative and endoplasmic reticulum stress, and tumor cell death via apoptosis, autophagy, pyroptosis, and necrosis. Viral replication culminates in cell lysis, releasing progeny viruses, tumor antigens, and danger signals (PAMPs and DAMPs) that convert immunologically “cold” tumors into inflamed environments. These signals are recognized by dendritic cells through toll-like receptors, triggering the release of type I interferons, TNF-a, and IL-2, which promote antigen presentation, Th1 polarization, activation of CD8? T cells and natural killer cells, and suppression of regulatory T cells. TNF-a enhances apoptosis, necrosis, and antiangiogenic effects, while IL-2 supports cytotoxic lymphocyte expansion. OVs can be naturally selective or genetically engineered to target tumor-associated transcription factors and surface markers while reducing pathogenicity in normal tissues. Appropriate delivery routes optimize tumor exposure, and combination with immune checkpoint inhibitors such as PD-L1 or CTLA-4 blockers further enhances viral persistence, immune activation, and systemic antitumor responses, including effects on metastatic disease.
Oncolytic viruses (OVs) are diverse anticancer agents with distinct mechanisms enabling tumor-selective targeting. Adenoviruses are widely used due to their nonintegrating genome and ease of genetic modification to enhance safety and efficacy. H-1 protoparvovirus is a nonpathogenic, self-replicating virus with early clinical activity in glioblastoma and pancreatic cancer. Herpes simplex virus type 1 is highly adaptable through virulence gene deletions, exemplified by approved therapies such as T-VEC. Vaccinia virus supports rapid replication and large transgene insertion, with Pexa-vec enhancing systemic antitumor immunity. Reovirus is a naturally oncolytic RNA virus that targets RAS-activated tumors and crosses the blood–brain barrier. Poliovirus, reengineered as PVSRIPO, selectively targets CD155-expressing tumors and induces durable immune responses in glioblastoma. Newcastle disease virus and picornaviruses, including Coxsackie virus, selectively infect cancer cells with defective antiviral defenses and are being developed to further improve tumor specificity and immune activation.
Oncolytic viruses (OVs) represent a promising cancer therapy by selectively destroying tumor cells while sparing normal tissue and are being investigated across multiple malignancies, including glioblastoma, melanoma, pancreatic, and ovarian cancers. They can be administered as monotherapies or in combination with chemotherapy, radiotherapy, or immunotherapy to enhance treatment efficacy. Through direct oncolysis and stimulation of antitumor immune responses, OVs can help overcome resistance to conventional therapies and improve clinical outcomes. Clinical trials have established their safety and demonstrated encouraging therapeutic potential, while ongoing research focuses on optimizing delivery, tumor specificity, and immune activation to maximize clinical benefit.
Although CT and MRI are valuable for tumor localization, their limited sensitivity restricts early detection of small or metastatic lesions. Engineered oncolytic viruses (OVs) offer a highly specific imaging strategy by selectively infecting tumor cells and expressing reporter genes such as luciferase, GFP, hNIS, TK, and hSSTR2, enabling real-time molecular and nuclear imaging. Preclinical studies using adenovirus, VSV, vaccinia, and measles viruses have demonstrated superior imaging accuracy through optical, PET, SPECT/CT, and MRI modalities. The ability to bioengineer OVs to enhance tumor targeting, immune activation, and reporter expression makes them powerful theranostic tools with strong potential for early cancer diagnosis and treatment monitoring.
Oncolytic viruses (OVs) selectively infect cancer cells, presenting tumor-associated antigens, activating danger signals to overcome immune suppression, and delivering therapeutic genes such as cytokines, immune checkpoint inhibitors, or pro-drug converting enzymes. The clinical success of genetically engineered OVs, exemplified by talimogene laherparepvec (T-VEC) for advanced melanoma, has demonstrated their safety, immunogenicity, and antitumor efficacy, spurring further development across cancers including glioblastoma, colorectal, and lung cancer. By lysing tumor cells and stimulating robust immune responses, OVs can counteract tumor-mediated immune evasion mechanisms such as impaired antigen presentation, checkpoint receptor dysregulation, and immunosuppressive macrophage activity. Their therapeutic potential is enhanced when combined with conventional treatments, immunotherapy, or targeted therapies. Ongoing research focuses on optimizing OV delivery, persistence, and tumor microenvironment modulation, positioning them as a versatile, multimodal strategy that integrates direct oncolysis with immune activation for improved cancer outcomes.
Recent Developments in the Oncolytic Virus Therapies Space
- In November 2025, Imugene Ltd and JW Therapeutics announced a co-development collaboration to evaluate the combination of Imugene's oncolytic virus CF33-CD19 (onCARlytics) and JW's Carteyva®, a CD19-directed autologous CAR-T cell therapy, for patients with advanced solid tumors. This collaboration includes preclinical in vitro and in vivo studies, followed by a Phase I investigator-initiated trial to be conducted exclusively in China at premier CAR-T clinical centers. The approach utilizes Imugene's CF33-CD19 virus to induce CD19 expression on tumor cells, rendering them susceptible to targeting by CD19 CAR-T therapies, a transformative ""mark and kill"" strategy.
- In July 2025, Calidi Biotherapeutics, Inc., a clinical-stage biotechnology company pioneering the development of targeted therapies with the potential to deliver genetic medicines to distal sites of disease, announced that it received Fast Track designation from the U.S. Food and Drug Administration (FDA) for CLD-201 (SuperNova), the company’s allogeneic adipose stem-cell loaded oncolytic virus for the treatment of soft tissue sarcoma.
- In June 2025, Oncolytics Biotech presented a poster demonstrating Role of pelareorep in activating anti-tumor immunity in PDAC.
- In May 2025, Candel Therapeutics, Inc. announced that the U.S. Food and Drug Administration (FDA) has granted Regenerative Medicine Advanced Therapy (RMAT) designation to CAN-2409 (aglatimagene besadenovec), the Company’s biological immunotherapy lead candidate, for the treatment of newly diagnosed localized prostate cancer in patients with intermediate-to-high-risk disease. CAN-2409 was also previously granted FDA Fast Track designation for the same indication.
- In May 2025, Elicera Therapeutics AB announced that it has entered into a Material Transfer Agreement (MTA) with University Hospital Tübingen in Germany. Under the agreement, the company’s oncolytic virus candidates ELC-100 and ELC-201 will be included in tests aimed at developing a new type of companion diagnostic to predict the potential success of a novel neuroendocrine neoplasm (NEN) therapy, immuno-virotherapy.
- In April 2025, Hangzhou ConVerd Co., Ltd announced that the first Phase IIa clinical study of hV01, ConVerd's first oncolytic vaccinia virus product, successfully completed its first patient dosing, with the subject in good condition. This phase IIa clinical study investigates the administration of a single dose of recombinant human IL-21 oncolytic poxvirus injection (hV01) per cycle for the treatment of patients diagnosed with advanced sarcoma and cervical cancer. It is important to note that the indication is for advanced sarcoma and recurrent or metastatic cervical cancer for which there is no standard of care available at this stage of the treatment process or where standard of care has failed.
Oncolytic Virus: Company and Product Profiles (Marketed Therapies)
1. Company Overview: Shanghai Sunway Biotech
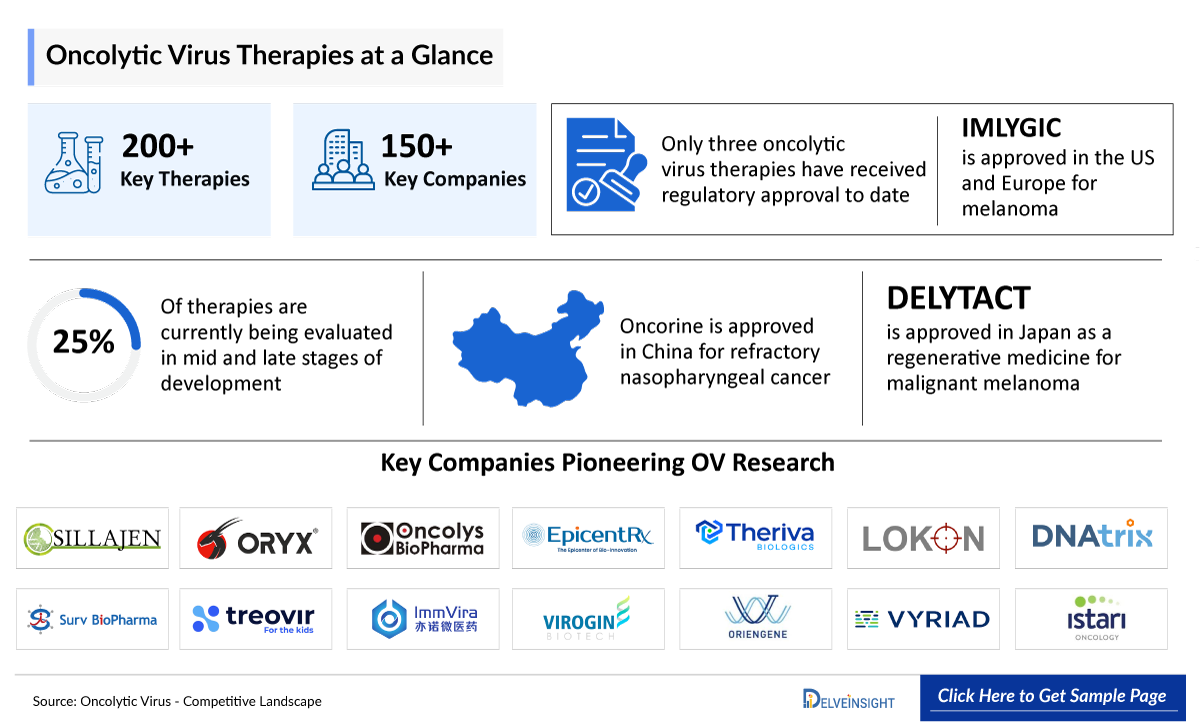
Shanghai Pharma Sunway Biotech Co., Ltd. (Sunway Bio), established in 1995 and based in the China (Shanghai) Pilot Free Trade Zone, is an innovation-driven biopharmaceutical company focused on gene therapy and immunotherapy drug development and industrialization. It specializes in cell and virus process R&D, virus culture and purification, pharmaceutical formulation, and quality testing, supported by comprehensive production and quality control capabilities. Sunway Bio notably owns the world’s first approved oncolytic virus therapy and aims to advance global healthcare by accelerating the development and manufacturing of innovative treatments through strong scientific expertise and industrialization platforms.
Product Description: Oncorine
Oncorine is a genetically modified adenovirus named H101 (E1B-deletion) developed by Shanghai Sunway Biotech in China, which is used in conjunction with chemotherapy for the treatment of nasopharyngeal carcinoma and head and neck cancer. The approval and successful application of Oncorine greatly promote the development of cancer immunotherapy based on the oncolytic virus. Oncorine, also referred to as Recombinant Human Adenovirus Type 5 Injection, was the first approved oncolytic virus by the Chinese State Food and Drug Administration (CFDA) for the cancer treatment in November 2005. Since its lauch, Oncorine has shown a good safety profile, and significant anti-tumor efficacy on a variety of tumors and malignant pleural effusion. Oncorine has been studied in exploratory clinical research for the treatment of liver malignancy, pancreatic cancer, cervical cancer, malignant melanoma, and metastatic liver cancer
Oncolytic Virus: Company and Product Profiles (Pipeline Therapies)
1. Company Overview: Candel Therapeutics
Candel Therapeutics is a clinical-stage biopharmaceutical company advancing off-the-shelf viral immunotherapies designed to trigger individualized, systemic antitumor immune responses by harnessing both innate and adaptive immunity against solid tumors. Its proprietary virus-based approaches aim to create “in situ immunization” by stimulating immune memory against tumor-specific antigens released during cancer cell lysis, with the goal of improving survival and quality of life across early to late disease stages. The company’s mission is to save, extend, and improve patient lives through these viral immunotherapies, and it leverages its scientific leadership to translate this vision into therapeutic candidates and clinical progress.
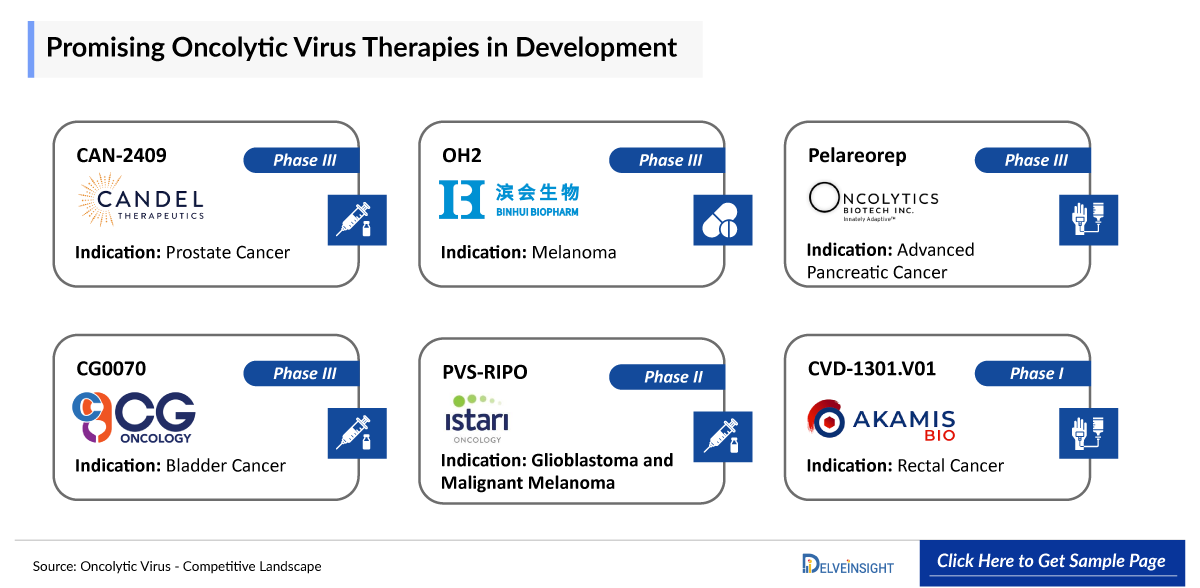
Product Description: CAN-2409
CAN-2409 is an investigational off-the-shelf replication-defective adenovirus designed to deliver the herpes simplex virus thymidine kinase (HSV-tk) gene to a patient’s specific tumor and induce an individualized, systemic immune response against the disease. CAN-2409 has the potential to treat a broad range of solid tumors. Encouraging monotherapy activity as well as combination activity with standard of care radiotherapy, surgery, chemotherapy, and ICI have previously been shown in several preclinical and clinical settings. CAN-2409 is based on Candel’s enLIGHTEN™ Discovery Platform which is a systematic, iterative HSV-based discovery platform leveraging human biology and advanced analytics to create new viral immunotherapies for solid tumors. Currently the drug is in Phase III stage of development for the treatment of Prostate Cancer.
2. Company Overview: Binhui Biopharmaceutical
Binhui Biopharmaceutical Co., Ltd. is a China-based clinical-stage biotechnology company headquartered in Wuhan, dedicated to developing innovative cancer immunotherapies with a focus on oncolytic virus-based treatments. Founded by Dr. Binlei Liu, a key contributor to the first FDA-approved oncolytic virus, the company leverages a proprietary platform using herpes simplex virus type 2 (oHSV2) to create therapies that selectively replicate in tumor cells and activate the immune system.
Product Description: OH2
Binhui Bio's lead drug, recombinant oncolytic herpes simplex virus BS001 (OH2) injection, employs advanced genetic engineering to remove neurotoxic and immunosuppressive genes while inserting immune-enhancing factors. OH2 selectively targets tumor cells, boosts antitumor immune responses, and enhances antigen presentation, offering a promising approach to cancer treatment. Currently the drug is in Phase III stage of development for the treatment of Melanoma.
3. Company Overview: Oncolytics Biotech
Oncolytics is a clinical-stage biotechnology company developing pelareorep, an investigational intravenously delivered double-stranded RNA immunotherapy designed to turn immunologically “cold” tumors “hot” by activating innate and adaptive immune responses. Pelareorep has shown promising results in pancreatic, breast, anal, and colorectal cancers and is being advanced in combination with chemotherapy and checkpoint inhibitors, with metastatic pancreatic and breast cancer programs receiving FDA Fast Track designation. The company is also pursuing strategic partnerships to accelerate development and enhance commercial potential.
Product Description: Pelareorep
Pelareorep is an oncolytic virus, which means that it preferentially lyses cancer cells. Pelareorep also promotes an inflamed tumor phenotype through innate and adaptive immune responses. Preliminary clinical trials indicate that it may have anti-cancer effects across a variety of cancer types (including breast, colorectal and pancreatic, as well as multiple myeloma) when administered alone and in combination with other cancer therapies. Currently the drug is in Phase III stage of development for the treatment of Advanced Pancreatic Cancer.
4. Company Overview: EpicentRx
EpicentRx is a San Diego-based biopharmaceutical company driven by a mission to transform how diseases are treated by developing innovative therapies and devices. The company strives to be a central force in advancing treatments that reduce both disease burden and treatment-related side effects, guided by the principle of “do no harm.” Founded to change how patients experience care, EpicentRx emphasizes minimal toxicity and maximum therapeutic activity in its approach to medical research and development.
Product Description: AdAPT-001
AdAPT-001 is an adenovirus that expresses a TGF-ß “trap”. This trap stalks and traps the TGF-ß cytokine hopefully for improved patient outcomes. Initial data from a Phase I/II with AdAPT-001 both alone and in combination with checkpoint inhibitors demonstrate that AdAPT-001 is well-tolerated with evidence of direct and abscopal tumor responses. The antitumor responses seen with checkpoint inhibitors in checkpoint inhibitor-resistant tumors suggest a mechanism of synergy between the two therapies. Currently the drug is in Phase II stage of development for the treatment of Sarcoma and Refractory Solid Tumors.
5. Company Overview: Hangzhou Converd
ConVerd, based in Hangzhou Future Sci-Tech City, is a pioneering biotech company specializing in oncolytic viruses and cancer immunotherapies. Backed by strategic investors, the company has built R&D platforms for next-generation oncolytic virotherapies and actively explores optimal combinations with other immunotherapies. ConVerd has also secured an extensive patent portfolio in cancer immunotherapy across China and beyond.
Product Description: CVD-1301.V01
CVD-1301.V01 is the first oncolytic vaccinia virus product independently innovated by Converd. The drug is the first clinical product of the tumor system immunotherapy product developed by the Converd for ten years, for the treatment of various solid tumors. The IND application for CVD-1301.V01, had been accepted by Center for Drug Evaluation, NMPA in December 2022. Currently the drug is in Phase II stage of development for the treatment of Advanced Pancreatic Cancer.
6. Company Overview: Imugene
Imugene is a clinical-stage immuno-oncology company committed to developing transformative cancer medicines that improve patients’ lives. Its mission emphasizes innovation driven by curiosity and creativity, a patient-centric focus where patients guide decision-making, and strong collaborative relationships with leading experts to advance cancer research. The company upholds ethical scientific integrity and strives for excellence across research, development, manufacturing, and operations.
Product Description: VAXINIA (CF33-hNIS)
CF33-hNIS is a novel chimeric DNA vaccinia virus engineered with the human sodium-iodide symporter (hNIS) gene. CF33-hNIS selectively replicates in tumor cells leading to cell lysis and the release of tumor- and virus-associated antigens stimulating antitumor immunity. CF33-hNIS has been tested in patients with gastrointestinal (GI) malignancies, including colorectal cancer, bile duct cancer, pancreatic cancer, and hepatocellular carcinoma. Currently, the drug is in the Phase I stage of its development for the treatment of solid tumors
Further product details are provided in the report……..
Oncolytic Virus Analytical Perspective by DelveInsight
In-depth Commercial Assessment: Oncolytic Virus Collaboration Analysis by Companies
The Report provides in-depth commercial assessment of drugs that have been included, which comprises collaboration, agreement, licensing and acquisition – deals values trends. The sub-segmentation is described in the report which provide company-company collaboration (licensing/partnering), company academic collaboration and acquisition analysis in tabulated form.
Oncolytic Virus Competitive Landscape
The report comprises of comparative assessment of Companies (by therapy, development stage, and technology).
Oncolytic Virus Report Assessment
- Company Analysis
- Therapeutic Assessment
- Pipeline Assessment
- Inactive drugs assessment
- Unmet Needs
Key Questions
Current Treatment Scenario and Emerging Therapies:
- How many companies are developing Oncolytic Virus drugs?
- How many Oncolytic Virus drugs are developed by each company?
- How many emerging drugs are in mid-stage, and late-stage of development for the treatment of Oncolytic Virus?
- What are the key collaborations (Industry–Industry, Industry–Academia), Mergers and acquisitions, licensing activities related to the Oncolytic Virus therapeutics?
- What are the recent trends, drug types and novel technologies developed to overcome the limitation of existing therapies?
- What are the clinical studies going on for Oncolytic Virus and their status?
- What are the key designations that have been granted to the emerging and approved drugs?