Schizophrenia Market Summary
- The Schizophrenia Market is expected to strengthen as awareness of the disease increases and more effective interventions are being developed.
- The Schizophrenia competitive landscape is undergoing dynamic changes with the approval of generic versions for drugs like LATUDA (lurasidone hydrochloride), leading to a substantial decline in its market revenue during the forecasted years.
Schizophrenia Market Insights and Epidemiological Forecast
- The Schizophrenia Prevalence Cases are projected to increase during the forecast period (2024–2034) due to the rising awareness regarding mental illness and improvements in diagnosis.
- During the initial phases of schizophrenia, symptoms lack specificity and coincide with other mental health issues or typical fluctuations in behavior. Schizophrenia Diagnosis is often delayed due to attached stigma and fear. This gap in the prevalent and diagnosed population of schizophrenia often leads to delays in initial treatment.
- At present, there is no cure for schizophrenia, and treatment primarily focuses on managing symptoms and preventing relapses. In some cases, hospitalization may be needed. The mainstay of treatment includes medication and psychosocial therapy that can help manage the condition. Pharmacological therapy plays a vital role in treating this disease, where many mono and combination pharmacological therapies are available for treating schizophrenia symptoms.
- Antipsychotic Drugs, integral to pharmacological therapy, dominate the market and are categorized into first and second generations. Notably, second-generation antipsychotics (SGA), comprised of the US FDA-approved medications, are favored over their first-generation (FGA) counterparts.
- The Schizophrenia Market is replete with antipsychotic drugs market diverse routes of administration (ROA), with a primary focus on dopamine modulation, establishing them as the conventional approach to treating schizophrenia. The US FDA-approved oral antipsychotics like CAPLYTA (lumateperone), VRAYLAR (cariprazine), LYBALVI (olanzapine and samidorphan), REXULTI (brexpiprazole), LATUDA (lurasidone hydrochloride), along with other oral therapies such as FANAPT and ABILIFY MYCITE.
- Recent research indicates that long-acting injectables (LAIs) may be more effective than oral medications in reducing the risk of hospitalization for schizophrenia. Injectables like ARISTADA|ARISTADA INITIO (aripiprazole lauroxil), ABILIFY MAINTENA (aripiprazole), INVEGA PRODUCTS, RISPERDAL CONSTA (risperidone), PERSERIS (risperidone), and other injectables play a significant role in this domain.
- Over the years, advancements in Schizophrenia Treatment have been notable, but non-adherence persists among patients, leading to increased morbidity and mortality. Although established atypical antipsychotics manage positive symptoms, their efficacy varies, and they have a limited impact on cognitive and negative symptoms.
- In 2022, the US had the largest Schizophrenia market size among the 7MM, accounting for approximately USD 5,749.0 million. This is expected to increase further by 2034.
- Significant increases in research and development, a better understanding of the disease, and technological advancements have helped broaden clinical knowledge. Ongoing research has led to the discovery of potential therapies that aim to address unmet needs. Schizophrenia emerging therapies like Sumitomo Pharma/Otsuka Pharmaceuticals’ Ulotaront (SEP-363856), Reviva Pharmaceuticals’ brilaroxazine (RP5063), and others are in development and are projected to enter the market during the forecast period, leading to the entry of new players in the Schizophrenia treatment market landscape.
- Brilaroxazine (RP5063) is a novel compound demonstrating strong affinity and selectivity for serotonin and dopamine receptors known to be involved in schizophrenia and other psychiatric conditions. Its anticipated introduction in the US market in 2026 is expected to significantly challenge existing therapies in the field.
- BMS’s novel medication, KarXT—now named COBENFY—received significant FDA approval on 26 September 2024 for the treatment of adults with schizophrenia. COBENFY is the first muscarinic agonist approved for schizophrenia, marking the first new treatment class for the condition since the FDA approved CLOZARIL (clozapine) 35 years ago.
Download the Sample PDF to Get More Insight @ Schizophrenia Treatment Market
Key Factors Driving the Schizophrenia Market Growth
-
Rising Schizophrenia Burden
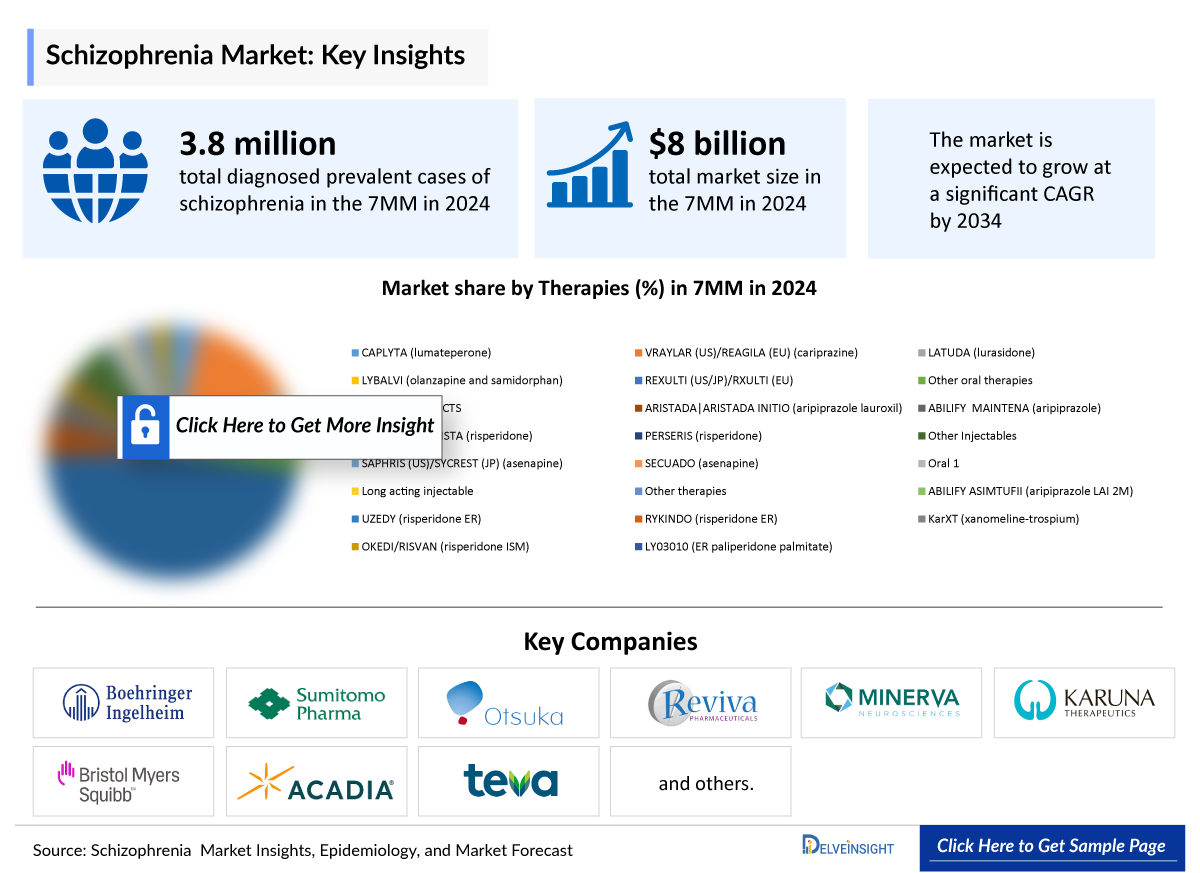
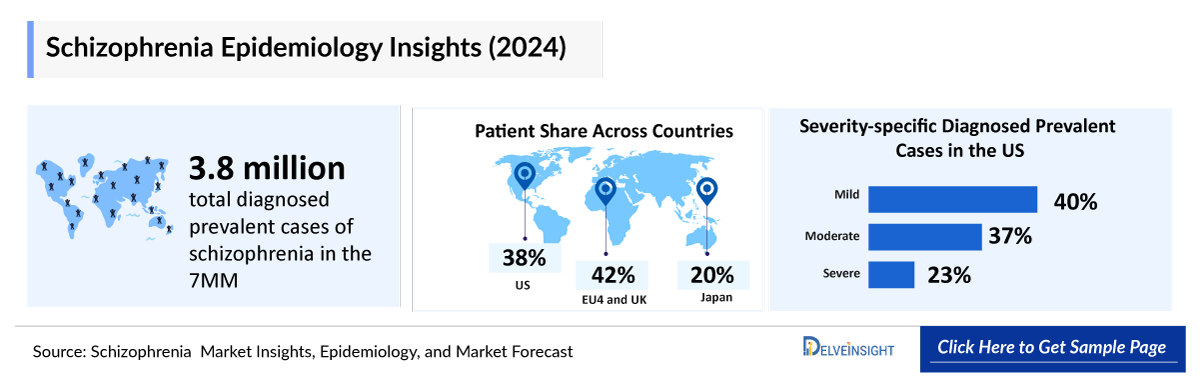
Schizophrenia continues to impose a significant public health challenge, with prevalence projected to rise in the coming decade. As per DelveInsight’s analysis, in 2024 the total diagnosed prevalent cases of schizophrenia in the 7MM were 3.8 million, of which the US accounted for nearly 1.4 million cases. These numbers are expected to grow by 2034, driven by advancements in psychiatric research and evolving diagnostic criteria, enabling more precise identification and earlier diagnosis of schizophrenia globally.
-
Schizophrenia Treatment Landscape
Second-generation antipsychotics, which dominate the treatment market, aim to address both positive and negative symptoms as well as neurocognitive impairments. These include REXULTI (brexpiprazole), CAPLYTA (lumateperone), LATUDA (lurasidone hydrochloride), VRAYLAR (cariprazine), ABILIFY MYCITE (aripiprazole with sensor), LYBALVI (olanzapine and samidorphan), SAPHRIS and SECUADO (asenapine), INVEGA SUSTENNA/TRINZA/HAFYERA (paliperidone palmitate), ARISTADA/ARISTADA INITIO (aripiprazole lauroxil), PERSERIS (risperidone), FANAPT (iloperidone), and others.
A major recent addition to the treatment arsenal is Bristol Myers Squibb’s COBENFY (KarXT), which received US FDA approval in September 2024.
-
Launch of Emerging Schizophrenia Drugs
The competitive landscape is set to expand further with several promising emerging therapies projected to launch during the forecast period. Key candidates include Teva/MedinCell/Royalty Pharma’s Olanzapine LAI (TEV-‘749), Ulotaront (SEP-363856; Sunovion/Otsuka), Reviva Pharmaceuticals’ Brilaroxazine (RP5063), Roluperidone (MIN-101; Minerva Neurosciences/Mitsubishi Tanabe), Valbenazine (NBI-98854; Neurocrine/Mitsubishi Tanabe), LYN-005 (long-acting oral risperidone; Lyndra Therapeutics), and Evenamide (NW-3509; Newron Pharmaceuticals). These therapies aim to improve long-term disease management, enhance adherence, and address unmet needs in treating negative and cognitive symptoms.
DelveInsight’s “Schizophrenia Market Insights, Epidemiology, and Market Forecast – 2034” report delivers an in-depth understanding of Schizophrenia, historical and forecasted epidemiology, as well as the Schizophrenia market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
The Schizophrenia Treatment Market report provides current treatment practices, emerging drugs, market share of individual therapies, and current and forecasted 7MM Schizophrenia market size from 2020 to 2034. The report also covers Schizophrenia treatment market practices/algorithms and Schizophrenia unmet needs to curate the best opportunities and assess the market’s potential.
Scope of the Schizophrenia Market | |
|
Study Period |
2020 to 2034 |
|
Forecast Period |
2024-2034 |
|
Geographies Covered |
|
|
Schizophrenia Drugs Market |
|
|
Schizophrenia Market Size | |
|
Schizophrenia Companies |
|
|
Schizophrenia Epidemiology Segmentation |
|
Schizophrenia Treatment Market
According to the World Health Organization (WHO), schizophrenia is a complex, long term and severe mental disorder characterized by a range of different psychological symptoms, including abnormalities in perception, thinking, emotions, and behavior. Contrary to public perception, schizophrenia is not a split or multiple personality. Besides, it is not caused by childhood experiences, poor parenting, or lack of willpower, nor are the symptoms identical in each patient. In addition, the vast majority of people with schizophrenia are not violent and do not pose a danger to others.
Schizophrenia is a severe mental illness affecting about 1% of Americans, impacting thinking, emotions, decision-making, and social interaction. Onset typically occurs in late teens to early 20s for men and late 20s to early 30s for women. Genetics and environmental factors contribute to its development, with a strong familial predisposition.
The schizophrenia risk factors include genetic and environmental factors, brain structure and function changes, and viral infections and immune disorders. Since multiple factors may contribute, scientists still do not have the exact cause of this in individual cases. Since the term schizophrenia embraces several different disorders, variation in the cause between cases is expected.
Schizophrenia symptoms vary but typically fall into three categories: psychotic (e.g., hallucinations and delusions), negative (e.g., loss of motivation and social withdrawal), and cognitive (e.g., attention and memory problems). Hallucinations involve perceiving things that are not real, such as hearing voices. Delusions are strong, false beliefs. Negative symptoms include loss of motivation and social withdrawal, while cognitive symptoms affect attention and memory, making daily functioning challenging.
Schizophrenia Diagnosis
Diagnosing schizophrenia is not easy. Sometimes using drugs, such as methamphetamines or LSD, can cause a person to have schizophrenia-like symptoms. The difficulty of diagnosing this illness is compounded by the fact that many people diagnosed do not believe they have it. Lack of awareness is a common symptom of schizophrenia and greatly complicates treatment.
While no single physical or lab test can diagnose schizophrenia, a healthcare provider who evaluates the symptoms and the course of a person’s illness over 6 months can help ensure a correct diagnosis. The health care provider must rule out other factors such as brain tumors, possible medical conditions, and other psychiatric diagnoses, such as bipolar disorder. To be diagnosed with schizophrenia, a person must have two or more of the following symptoms occurring persistently in the context of reduced functioning: delusions, hallucinations, disorganized speech, disorganized or catatonic behavior, and negative symptoms.
Further details related to country-based variations are provided in the report…
Schizophrenia Treatment
Schizophrenia is a lifelong condition, but effective treatment can help a person manage the symptoms, prevent relapses, and avoid hospitalization. Schizophrenia requires lifelong treatment, even when symptoms have subsided. Treatment with medications and psychosocial therapy can help manage the condition; in some cases, hospitalization may be needed. A psychiatrist experienced in treating schizophrenia guides treatment. The treatment team also may include a psychologist, social worker, psychiatric nurse, and possibly a case manager to coordinate care. The full-team approach may be available in clinics with expertise in schizophrenia treatment.
Schizophrenia requires a combination of treatments, including medication (antipsychotics), psychological counseling and social support, cognitive behavioral therapy, and electroconvulsive therapy (ECT). Currently, approved pharmacologic agents focus on modulating dopamine, leaving patients with schizophrenia to cope with considerable residual symptoms. Suboptimal treatment, significant AEs, and challenges related to nonadherence need new agents to manage schizophrenia better.
Electroconvulsive therapy and hospitalization may be necessary for those who do not respond to drug therapy or during severe episodes.
Schizophrenia Epidemiology
As the market is derived using a patient-based model, the Schizophrenia epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by total prevalent cases of schizophrenia, total diagnosed prevalent cases of schizophrenia, gender-specific diagnosed prevalent cases of schizophrenia, and severity-specific diagnosed prevalent cases of schizophrenia in the 7MM covering the United States, EU4 countries (Germany, France, Italy, and Spain) and the United Kingdom, and Japan from 2020 to 2034.
- In the 7MM, the total Schizophrenia prevalence cases were estimated to be approximately 6,029,994 in 2022, of which the US accounted for around 45.05%, while EU4 and the UK accounted for nearly 37.36%, and Japan accounted for approximately 17.59% of the total prevalent cases.
- Among the 7MM, the US accounted for nearly 37.20% of the total Schizophrenia diagnosed prevalent cases with nearly 1,412,470 cases in 2022. These cases are expected to increase during the study period (2020–2034).
- As per DelveInsight analysis, EU4 and the UK accounted for around 1,588,816 Schizophrenia diagnosed prevalent cases in 2022. These cases are expected to change during the study period (2020–2034)
- Among the EU4 and the UK, Germany accounted for the highest Schizophrenia prevalence cases representing nearly 28.50% of the cases, followed by the UK, and France, while Spain had the least cases in 2022
- According to estimates based on DelveInsight’s epidemiology model, Schizophrenia exhibits a higher male preponderance than females in the US. Of the total diagnosed prevalent cases in the US, nearly 53.63% were males and 46.37% were females, in 2022.
- In the US, when the Schizophrenia diagnosed prevalent cases were segmented based on severity, the mild group accounted for nearly 567,336 cases, in 2022. As per the analysis, these cases are expected to increase, and the moderate group contributed around 37.03% of the diagnosed prevalent cases, while the severe group contributed a minimum of 22.81% respectively in 2022.
- In 2022, among the 7MM, Japan had the second highest cases of Schizophrenia, contributing approximately 17.59% to the total prevalent cases of Schizophrenia.
Schizophrenia Market Recent Developments
- In October 2025, Celon Pharma received FDA clearance to proceed with Phase 3 trials of CPL’36, a second-generation PDE10A inhibitor for schizophrenia. The FDA confirmed existing preclinical data support pivotal studies, including two 28-day randomized trials and a 12-month safety extension.
- In September 2025, Lupin Limited announced FDA approval of its Abbreviated New Drug Application (ANDA) for Risperidone extended-release injectable suspension in single-dose vials of 25 mg, 37.5 mg, and 50 mg. This is Lupin’s first product using proprietary Nanomi B.V. technology and carries a 180-day exclusivity. Nanomi, a Lupin subsidiary, focuses on developing innovative long-acting injectable medicines to enhance patient outcomes.
- In Sept 2025, Amneal Pharmaceuticals received FDA approval for risperidone extended-release injectable suspension (12.5 to 50 mg/vial), a treatment for schizophrenia and Bipolar I disorder. The product, referencing Janssen’s Risperdal Consta®, qualifies for 180-day exclusivity under the FDA’s CGT designation and is set to launch in Q4 2025.
- In Sept 2025, Lupin Limited received FDA approval for its ANDA of Risperidone extended-release injectable suspension in 25 mg, 37.5 mg, and 50 mg single-dose vials. This marks Lupin’s first product using proprietary Nanomi B.V. technology, with a 180-day exclusivity period. Nanomi, a Lupin subsidiary, specializes in developing innovative long-acting injectable medicines.
- In August 2025, BioXcel Therapeutics announced that it received positive pre-sNDA meeting responses from the FDA, confirming that the planned regulatory package for BXCL501 is sufficient to support a supplemental NDA submission, expected in Q1 2026.
- In August 2025, Boehringer Ingelheim and Click Therapeutics announced that the Phase III CONVOKE trial of CT-155 (BI 3972080), an investigational prescription digital therapeutic (PDT), met its primary endpoint in improving negative symptoms of schizophrenia when used alongside standard antipsychotic therapy.
- In July 2025, EuMentis Therapeutics announced FDA authorization of its Investigational New Drug (IND) application to begin a Phase 2 trial of EM-221, a PDE10A inhibitor, for treating schizophrenia.
- In July 2025, BioXcel Therapeutics submitted a pre-sNDA meeting package to the FDA for IGALMI®, aiming to expand its label for outpatient (at-home) use in treating acute agitation associated with bipolar disorders and schizophrenia. The meeting is scheduled for August 20, 2025.
- In May 2025, Vanda Pharmaceuticals announced that the FDA has filed the New Drug Application (NDA) for Bysanti™ (milsaperidone), with no potential review issues identified. The FDA set February 21, 2026, as the target date for its decision. Milsaperidone, a new chemical entity, is an active metabolite of iloperidone, and clinical studies have shown bioequivalence between the two at various doses.
- In April 2025, CHA Biotech's subsidiary CMG Pharmaceutical received FDA approval for Mezofy (formerly Depipzo), an oral film-type treatment for schizophrenia. Mezofy, containing aripiprazole, addresses patient non-compliance by dissolving easily in the mouth, eliminating the need for water.
- In March 2025, Vanda Pharmaceuticals Inc. announced the submission of a New Drug Application (NDA) to the FDA for marketing approval of Bysanti™ (milsaperidone) for the treatment of acute bipolar I disorder and schizophrenia. The NDA is supported by multiple clinical studies evaluating Bysanti™'s efficacy and safety.
- In January 2025, Boehringer announced that its Phase III CONNEX program for the investigational schizophrenia drug iclepertin did not meet its primary or key secondary endpoints. After six months, the drug showed no significant cognitive or functional improvements compared to placebo in any of the three studies.
-
In January 2025, Johnson & Johnson and Intra-Cellular Therapies announced a definitive agreement under which Johnson & Johnson will acquire all outstanding shares of Intra-Cellular Therapies, a biopharmaceutical company specializing in the development and commercialization of CNS disorder treatments, for $132.00 per share in cash, totaling approximately $14.6 billion in equity value.
- In November 2024, AbbVie announced that its two Phase 2 EMPOWER trials of emraclidine, a once-daily oral monotherapy for adults with schizophrenia experiencing acute psychotic symptoms, did not meet the primary endpoint. The trials showed no statistically significant improvement in the Positive and Negative Syndrome Scale (PANSS) total score compared to placebo at week 6.
- In September 2024, Bristol Myers Squibb (BMY) announced that the U.S. Food and Drug Administration (FDA) approved COBENFY™ (xanomeline and trospium chloride), an oral medication for treating schizophrenia in adults. The FDA approval is especially notable as it marks the first novel drug for schizophrenia in 35 years, introducing a new class of treatment by selectively targeting M1 and M4 receptors in the brain without blocking D2 receptors. This breakthrough has the potential to reshape the treatment landscape for schizophrenia, a milestone highlighted by CEO Chris Boerner, PhD, who emphasized the significance of this novel approach in neuropsychiatry.
- In September 2024, Teva Pharmaceuticals announced positive results from its Phase 3 SOLARIS trial for TEV-749, a subcutaneous injection for schizophrenia. The trial demonstrated significant reductions in the Positive and Negative Syndrome Scale (PANSS) total score by week 8, along with improvements in key secondary endpoints. Notably, there were no cases of post-injection delirium/sedation syndrome (PDSS). These findings were presented at the 37th Annual European College of Neuropsychopharmacology Congress in Milan, Italy, from September 21-24, 2024.
- In September 2024, BMO Capital Markets analyst Evan Seigerman raised concerns about Neurocrine Biosciences’ developmental strategy following inconsistent results from mid-stage trials of luvadaxistat. Neurocrine announced it will discontinue development of the investigational schizophrenia drugs after the Phase II ERUDITE study failed to meet its primary endpoint of cognitive improvement in over 200 patients. The company noted that luvadaxistat could not replicate the cognitivea benefits observed in the earlier INTERACT study, where a 50-mg dose showed significant cognitive improvements.
Schizophrenia Drugs Analysis
The drug chapter segment of the Schizophrenia Treatment Market report encloses a detailed analysis of Schizophrenia-marketed drugs and mid to late-stage (Phase III and Phase II) Schizophrenia Pipeline Drugs analysis. It also helps to understand the Schizophrenia clinical trials details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug, and the latest Schizophrenia news and press releases.
Schizophrenia Marketed Drugs
-
ABILIFY MAINTENA (aripiprazole): Otsuka Pharmaceutical/Lundbeck
ABILIFY MAINTENA (aripiprazole) for extended-release injectable suspension, an IM depot formulation of aripiprazole, is a sterile lyophilized powder that, when reconstituted with sterile water for injection, forms an injectable suspension that can be administered monthly. ABILIFY MAINTENA is an atypical antipsychotic drugs market. The mechanism of action of aripiprazole in the treatment of schizophrenia and bipolar I disorder is unknown. The efficacy of aripiprazole could be mediated through a combination of partial agonist activity at dopamine D2 and serotonin 5-HT1A receptors and antagonist activity at 5-HT2A receptors.
In March 2013, the FDA approved ABILIFY MAINTENA (aripiprazole) for extended-release injectable suspension, an intramuscular depot formulation for treating schizophrenia. In September 2014, the FDA approved a pre-filled dual-chamber syringe for ABILIFY MAINTENA. In December 2014, the FDA approved the labeling update of ABILIFY MAINTENA for treating acutely relapsed adults with schizophrenia. In November 2013, the EMA approved ABILIFY MAINTENA (aripiprazole) for extended-release injectable suspension–an intramuscular depot formulation indicated for treating schizophrenia, and in March 2015, ABILIFY received regulatory approval in Japan for the manufacture and marketing of ABILIFY in 300 mg and 400 mg vials and in 300 mg and 400 mg dual-chamber syringe extended-release Injectable Suspension.
-
UZEDY (risperidone): Teva Pharmaceuticals/MedinCell
UZEDY (risperidone) extended-release injectable suspension is indicated for the treatment of schizophrenia in adults. UZEDY administers risperidone through copolymer technology under license from MedinCell that allows for absorption and sustained release in the first subcutaneous injection. UZEDY is the only long-acting, subcutaneous formulation of risperidone available in both one- and two-month dosing intervals.
UZEDY is the first subcutaneous, long-acting formulation of risperidone that utilizes SteadyTeq, a copolymer technology proprietary to MedinCell that controls the steady release of risperidone. UZEDY (risperidone) extended-release injectable suspension was approved by the FDA in April 2023 for the treatment of schizophrenia in adults and launched in the US in May 2023.
Note: Further marketed drugs and their details will be provided in the report…
Schizophrenia Emerging Drugs
-
Ulotaront (SEP-363856): Sumitomo Pharma/Otsuka Pharmaceuticals
Ulotaront (SEP-363856) is a trace amine-associated receptor 1 (TAAR1) agonist with serotonin 5-HT1A agonist activity, jointly developed by Sunovion Pharma and PsychoGenics, which is a small-molecule oral agent that does not bind to dopamine D2 or serotonin 5-HT2A receptors. Sunovion discovered Ulotaront in collaboration with PsychoGenics using it is in vivo phenotypic SmartCube platform and associated artificial intelligence algorithms.
In May 2019, the US FDA granted BTD for SEP-363856 for the treatment of people with schizophrenia.
-
Brilaroxazine (RP-5063): Reviva Pharmaceuticals
Brilaroxazine (RP5063) is a new chemical entity with potent affinity and selectivity against key serotonin and dopamine receptors implicated in schizophrenia and other psychiatric disorders. RP5063 is a multimodal modulator of the serotonin 5-HT1A, 5-HT2A, 5-HT2B, and 5-HT7 receptors and D2, D3, and D4 dopamine receptors in clinical development for multiple neuropsychiatric indications, including schizophrenia.
Recently, the company posted topline results and the successful completion of a pivotal Phase III RECOVER trial evaluating the efficacy, safety, and tolerability of once-daily RP5063 in adults with acute schizophrenia.
Note: Further emerging therapies and their detailed assessment will be provided in the final report.
Schizophrenia Drugs Market Insights
Schizophrenia treatment typically involves a combination of medication and psychosocial interventions aimed at managing the condition effectively. Pharmacotherapy plays a pivotal role in addressing schizophrenia symptoms, with a wide array of mono and combination pharmacological options available. Antipsychotic medications, categorized into first-generation (FGAs) and second-generation (SGAs), are commonly prescribed as first-line treatments. Some treatment approaches may involve a combination of antipsychotics and antiepileptic medications, tailored to specific patient groups. Both oral antipsychotics (OAP) and long-acting injectable therapies (LAI) are accessible options within each generation of antipsychotics drugs market. In clinical practice, a blend of both FGAs and SGAs is often utilized to effectively manage schizophrenia symptoms.
First-generation antipsychotics (FGAs) encompass medications such as chlorpromazine, fluphenazine, haloperidol, and perphenazine. FGAs are associated with frequent and potentially significant neurological side effects, including the risk of developing tardive dyskinesia, a movement disorder that may or may not be reversible. Consequently, second-generation antipsychotic (SGA) medications are generally preferred due to their lower propensity for serious side effects compared to FGAs. SGAs, including REXULTI/RXULTI (brexpiprazole), CAPLYTA (lumateperone), LATUDA (lurasidone hydrochloride), SAPHRIS (asenapine), ABILIFY MYCITE (aripiprazole tablets with sensor), VRAYLAR/REAGILA (cariprazine), SECUADO (asenapine), INVEGA SUSTENNA/TRINZA/HAYFERA (paliperidone palmitate), ARISTADA/ARISTADA INITIO (aripiprazole lauroxil), PERSERIS (risperidone), FANAPT (iloperidone), LYBALVI (olanzapine and samidorphan), among others, are generally favored due to their lower risk of side effects.
Each of these medications brings a unique contribution to the evolving Schizophrenia treatment market landscape options, addressing specific needs and considerations in the complex realm of schizophrenia therapeutics.
Brexpiprazole, marketed under the brand name REXULTI, is an antipsychotic medication used in the Schizophrenia treatment. It works by modulating the activity of certain neurotransmitters in the brain, specifically dopamine and serotonin receptors. Brexpiprazole is classified as a second-generation antipsychotic (SGA) and is believed to exert its therapeutic effects through a combination of partial agonism and antagonism at dopamine D2 and serotonin 5-HT1A receptors, as well as antagonism at serotonin 5-HT2A receptors.
CAPLYTA (lumateperone) is another antipsychotic medication used in the treatment of schizophrenia. It operates through a distinct mechanism of action compared to traditional antipsychotics. Lumateperone is classified as a novel, investigational agent belonging to the class of compounds known as serotonin-dopamine activity modulators (SDAMs). In schizophrenia, lumateperone helps to alleviate symptoms such as hallucinations, delusions, disorganized thinking, and negative symptoms (such as social withdrawal or lack of motivation). Its unique mechanism of action may provide benefits in terms of efficacy and tolerability compared to traditional antipsychotic medicine market.
Schizophrenia Market Outlook
Schizophrenia is a severe and chronic mental disorder characterized by distorted thinking, perception, emotions, language, sense of self, and behavior. Its impact extends beyond patients to family members, caregivers, and society due to its lifelong nature and the likelihood of relapses. The disorder progresses through stages, including prodromal, active, and residual, each marked by specific symptoms such as hallucinations, suspiciousness, delusions, depression, withdrawal, anxiety, and difficulty concentrating. The treatment options for schizophrenia include antipsychotics medicine market, psychological counseling and social support, cognitive behavioral therapy, and electroconvulsive therapy (ECT).
The Schizophrenia Market is replete with antipsychotic drugs utilizing diverse routes of administration (ROA), with a primary focus on dopamine modulation, establishing them as the conventional approach to treating schizophrenia. FDA-approved oral antipsychotics medicine market include CAPLYTA (lumateperone), VRAYLAR (cariprazine), LYBALVI (olanzapine and samidorphan), REXULTI (brexpiprazole), LATUDA (lurasidone hydrochloride), along with other oral therapies such as FANAPT and ABILIFY MYCITE.
The Schizophrenia competitive landscape is undergoing dynamic changes with the approval of generic versions of drugs like LATUDA (lurasidone hydrochloride) and REXULTI. The current pharmacologic options for schizophrenia, primarily targeting positive symptoms through dopamine modulation, face challenges in addressing the full spectrum of the disorder. Non-adherence remains a critical issue, and there is a clear need for innovative treatments that can effectively manage both positive and negative symptoms, offering a more comprehensive approach to schizophrenia care.
At present, various Schizophrenia Companies have embarked on clinical trials to explore novel treatment options for schizophrenia. Prominent industry players, including Boehringer Ingelheim, Karuna Therapeutics, Acadia Pharmaceuticals, Reviva Pharmaceuticals, Minerva Neurosciences, and Newron Pharmaceuticals, among others, are advancing their products through the late phases of clinical development. The schizophrenia therapeutics market landscape is undergoing a notable influx of potential emerging drugs, each presenting unique mechanisms and strategic approaches in the quest for more effective treatment options. This dynamic scenario reflects a concerted effort within the industry to address the complexities of schizophrenia through innovative and diverse therapeutic interventions.
Continued in report…
The current Schizophrenia drugs market segmentation is based on the therapies prescribed. The drugs that are being used in the present market include CAPLYTA (lumateperone), VRAYLAR (US)/REAGILA (EU) (cariprazine), LATUDA (lurasidone), REXULTI (US/JP)/RXULTI (EU), and others are included. These are the major segments covered in the forecast model.
Several Schizophrenia Companies are evaluating their lead candidates in different stages of clinical development like Ulotaront (SEP-363856) by Sumitomo Pharma/Otsuka Pharmaceuticals. The market for Schizophrenia is expected to experience positive growth.
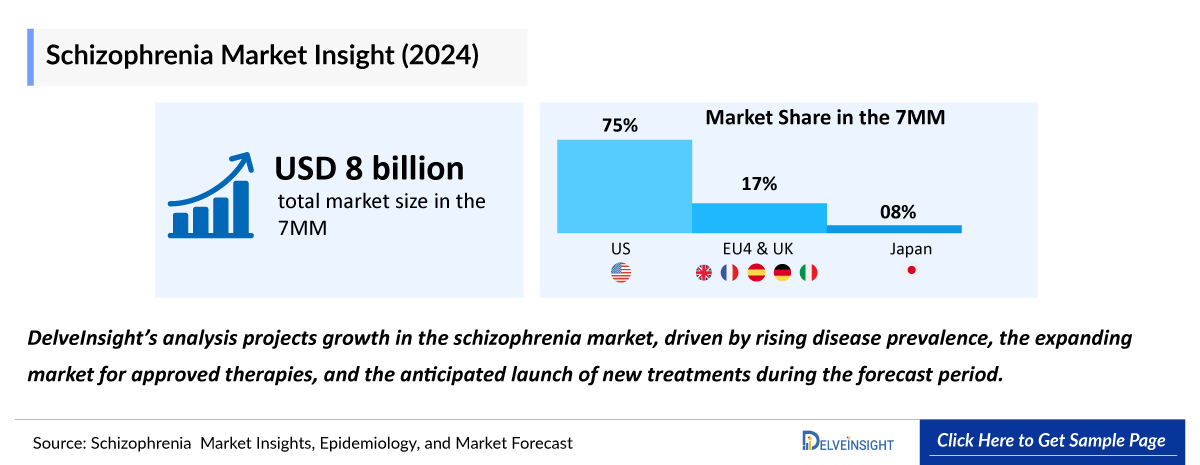
- The total Schizophrenia Market Size in the 7MM was approximately USD 7,972.0 million in 2022 and is projected to increase during the forecast period (2024–2034).
- The Schizophrenia market size in the US was approximately USD 5,749.0 million in 2022, which is anticipated to increase due to the increasing awareness of the disease and the launch of the emerging therapy.
- The Schizophrenia Market Size of EU4 and the UK was calculated to be approximately USD 1,547.1 million in 2022, which was nearly 19.41% of the total market revenue for the 7MM.
- According to DelveInsight’s estimates, among EU4 and the UK, Germany accounted for the highest market with approximately USD 432.0 million in 2022, followed by the UK with approximately USD 389.4 million in the respective year, while Spain accounted for the lowest market in 2022.
- According to DelveInsight’s analysis, in the US, among the currently used therapies, VRAYLAR, generated a revenue of approximately USD 772.4 million, while INVEGA Products, which includes INVEGA SUSTENNA, INVEGA TRINZA, and INVEGA HAFYERA (paliperidone palmitate) generated a revenue of USD 2,686.3 million, in 2022, followed by other therapies.
- In 2022, Japan with a revenue of approximately 675.9 million, which was nearly 8.48% of the total market revenue for the 7MM, which is expected to increase significantly by 2034.
Schizophrenia Drugs Uptake
This section focuses on the uptake rate of potential Schizophrenia drugs expected to be launched in the market during 2020–2034. For example, Sumitomo Pharma and Otsuka’s Ulotaront (SEP-363856), a trace amine-associated receptor 1 (TAAR1) agonist with serotonin 5-HT1A agonist activity, jointly developed by Sunovion Pharma and PsychoGenics is expected to enter the US market in 2028 with a “slow-medium” uptake.
Schizophrenia Pipeline Development Activities
The Schizophrenia therapeutics market report provides insights into different therapeutic candidates in Phase III, Phase II, and Phase I. It also analyzes Schizophrenia Companies involved in developing targeted therapeutics. The Schizophrenia therapeutics market report covers information on collaborations, acquisitions and mergers, licensing, and patent details for Schizophrenia emerging therapies
Latest KOL Views on Schizophrenia
To keep up with current Schizophrenia market trends, we take KOLs and SMEs’ opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts contacted for insights on evolving Schizophrenia's treatment market landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake, along with challenges related to accessibility, including Medical/scientific writers, Medical Professionals, Professors, Directors, and Others.
DelveInsight’s analysts connected with 50+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. Centers like the American Psychiatric Association, US, University of Maryland School of Medicine, Baltimore, US, Department of Psychiatry and Behavioral Medicine, Louisiana State University, University of Lyon, Lyon, France, University of Bari Aldo Moro, Italy, and National Center of Neurology and Psychiatry, Tokyo, Japan were contacted. Their opinion helps understand and validate current and emerging therapy treatment patterns or Schizophrenia market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
Physician’s View
According to our primary research analysis, despite advancements in the treatment of schizophrenia, significant unmet needs persist in effectively managing this complex disorder. One primary challenge lies in achieving optimal symptom control and functional recovery for all individuals, as responses to existing therapies can vary widely among patients. Additionally, while antipsychotic medicine market remain the cornerstone of treatment, many individuals experience inadequate efficacy or intolerable side effects, highlighting the need for novel therapeutic options with improved efficacy and tolerability profiles.
Furthermore, addressing the cognitive deficits and negative symptoms associated with schizophrenia, which often contribute to long-term disability and impaired quality of life, remains a major challenge. Additionally, there is a need for interventions that target the underlying neurobiological mechanisms of the disorder, as current treatments primarily address symptom management rather than disease modification. Enhancing access to evidence-based psychosocial interventions and support services, particularly in community settings, is also crucial for promoting long-term recovery and reducing the burden of schizophrenia on individuals, families, and society.
According to a KOL in the US, there are no therapies that can effectively address the complex combination of positive and negative symptoms, mood, and cognitive impairment associated with schizophrenia. As a result, emerging therapies have focused on developing a drug that targets both the serotonin and dopamine receptor signaling systems in order to treat schizophrenia and its comorbid symptoms more effectively.
As per another KOL, all persons with schizophrenia need drugs some of the time and most will do better with continued use of medication to help control symptoms and prevent relapse. However, the drugs are not effective for all aspects of the illness. Cognitive behavioral therapy may help with certain symptoms, and supportive psychotherapy can support personal strengths and improve quality of life.
Another KOL found that lack of efficacy is a common cause of treatment discontinuation. Although available antipsychotics can alleviate positive symptoms by blocking striatal dopamine D2 receptors, a significant number of patients fail to improve psychotic symptoms with these agents, as do rates of treatment resistance in first-episode patients compared to a community sample of chronic patients.
Schizophrenia Qualitative Analysis Report
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving Schizophrenia Treatment Market Landscape.
Conjoint Analysis analyzes multiple emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy. In efficacy, the trial’s primary and secondary outcome measures are evaluated; for instance Schizophrenia Incidence of overall adverse events (AEs), serious adverse events (SAEs), and AEs leading to discontinuation, and others.
Further, the therapies’ safety is evaluated wherein the adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials, which directly affects the safety of the molecule in the upcoming trials. It sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the route of administration, order of entry and designation, probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Schizophrenia Market Access and Reimbursement
The high cost of therapies for the treatment is a major factor restraining the growth of the drug market. Because of the high cost, the economic burden is increasing, leading the patient to escape from proper treatment. The reimbursement challenges related to medical care and treatment for individuals with Schizophrenia can be significant as it often requires specialized medical attention, covering the costs of diagnosis, treatment, and ongoing care.
Health insurance plans may not fully cover limited coverage of some medical treatments, and therapies specific to Schizophrenia. This can result in high out-of-pocket expenses for families seeking the best care for their loved ones. Moreover, it requires specialized care from healthcare providers with expertise. Finding and accessing such specialists may be challenging, and the associated costs may not always be fully reimbursed by insurance.
Further details will be provided in the report.
The Schizophrenia therapeutics market report provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenarios, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Schizophrenia Pipeline Drugs Market Report Scope
- The Schizophrenia Treatment Market report covers a segment of key events, an executive summary, and a descriptive overview, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight into the Schizophrenia epidemiology segments and forecasts, the future growth potential of diagnosis rate, disease progression, and treatment guidelines have been provided.
- Additionally, an all-inclusive account of the current and Schizophrenia emerging therapies and the elaborative profiles of late-stage and prominent therapies will impact the current Schizophrenia treatment market landscape.
- A detailed review of the Schizophrenia, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The patient-based Schizophrenia market forecasting report provides an edge while developing business strategies by understanding trends through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help shape and drive the 7MM Schizophrenia pipeline drugs market.
Schizophrenia Pipeline Drugs Market Report Insights
- Patient-based Schizophrenia Market Forecasting
- Therapeutic Approaches
- Schizophrenia Pipeline Drugs Analysis
- Schizophrenia Market Size
- Schizophrenia Market Trends
- Existing and Future Market Opportunity
Schizophrenia Pipeline Drugs Market Report Key Strengths
- 12 years Schizophrenia Market Forecast
- The 7MM Coverage
- Schizophrenia Epidemiology Segmentation
- Key Cross Competition
- Attribute analysis
- Schizophrenia Drugs Uptake
- Key Schizophrenia Market Forecast Assumptions
Schizophrenia Treatment Market Report Assessment
- Current Schizophrenia Treatment Market Practices
- Schizophrenia Unmet Needs
- Schizophrenia Pipeline Drugs Profiles
- Schizophrenia Pipeline Drugs Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
Key Questions Answered in the Schizophrenia Market Report
Schizophrenia Treatment Market Insights
- What was the total Schizophrenia market size, the Schizophrenia treatment market size by therapies, and Schizophrenia drugs market share (%) distribution in 2020, and what would it look like by 2034? What are the contributing factors for this growth?
- How will Ulotaront (SEP-363856), Brilaroxazine (RP5063), and others affect the treatment paradigm of Schizophrenia?
- How will Ulotaront (SEP-363856) compete with upcoming products and marketed therapies?
- Which drug is going to be the largest contributor by 2034?
- What are the pricing variations among different geographies for approved and marketed therapies?
- How would future opportunities affect the market dynamics and subsequent analysis of the associated trends?
Schizophrenia Epidemiology Insights
- What are the disease risks, burdens, and Schizophrenia Unmet Needs? What will be the growth opportunities across the 7MM with respect to the patient population pertaining to Schizophrenia?
- What is the historical and forecasted Schizophrenia patient pool in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan?
- Out of the countries mentioned above, which country would have the highest diagnosed prevalent Schizophrenia population during the forecast period (2024–2034)?
- What factors are contributing to the growth of Schizophrenia cases?
Current Schizophrenia Treatment Market Scenario, Marketed Drugs, and Emerging Therapies
- What are the current options for the Schizophrenia treatment? What are the current clinical and treatment guidelines for treating Schizophrenia?
- How many Schizophrenia companies are developing therapies for Schizophrenia treatment?
- How many emerging therapies are in the mid-stage and late stage of development for treating Schizophrenia?
- What are the recent novel therapies, targets, Schizophrenia mechanisms of action, and technologies developed to overcome the limitations of existing therapies?
- What is the cost burden of current Schizophrenia treatment on the patient?
- Patient acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the accessibility issues of approved therapy in the US?
- What is the 7MM historical and forecasted Schizophrenia Pipeline Drugs Market?
Reasons to Buy the Schizophrenia Market Report
- The Schizophrenia pipeline drugs market report will help develop business strategies by understanding the latest trends and changing treatment dynamics driving the Schizophrenia market.
- Insights on patient burden/disease Schizophrenia prevalence, evolution in Schizophrenia diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- Understand the existing Schizophrenia pipeline drugs market opportunities in varying geographies and the growth potential over the coming years.
- The distribution of historical and current patient share is based on real-world prescription data in the US, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
- Identifying upcoming solid Schizophrenia companies in the Schizophrenia pipeline drugs market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the conjoint analysis section to provide visibility around leading classes.
- Highlights of Access and Reimbursement policies for Schizophrenia, barriers to accessibility of approved therapy, and patient assistance programs.
- To understand Key Opinion Schizophrenia Companies perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing Schizophrenia pipeline drugs market so that the upcoming players can strengthen their development and launch strategy.
Stay Updated with Us for New Articles
- Evenamide (NW-3509): Advancing Solutions for Treatment-Resistant Schizophrenia
- Breaking Boundaries: Innovations and Updates in Schizophrenia Treatment
- How are Antipsychotics Transforming the Schizophrenia Treatment Space?
- Ulotaront (SEP-363856): A New Era in Schizophrenia Treatment
- Schizophrenia Market: Infographics
- Schizophrenia Newsletter
- Latest DelveInsight Blogs




.png&w=3840&q=75)
