Traumatic Brain Injury Market Summary
- The Traumatic Brain Injury market size is projected to expand from a valuation of USD 30 billion in 2024, registering a CAGR of 10.9% through 2034 across major markets, including the US, EU4, the UK, and Japan.
- The Traumatic Brain Injury companies developing therapies in the market include - Oxeia Biopharmaceuticals, Hope Biosciences, Oragenics, SHINKEI Therapeutics, BioVie, Beyond Barriers Therapeutics, AivoCode, Abliva, Owl Therapeutics, Neuroplast and others.
Traumatic Brain Injury Market and Epidemiology Analysis
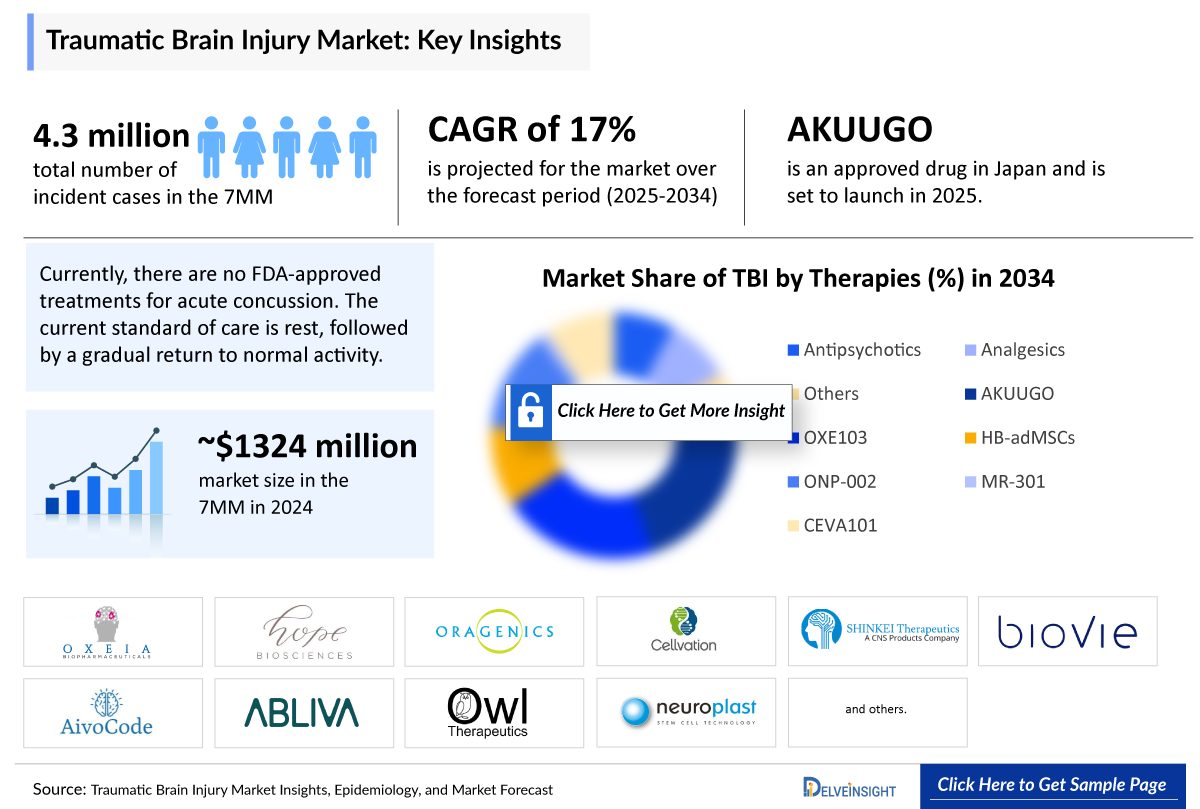
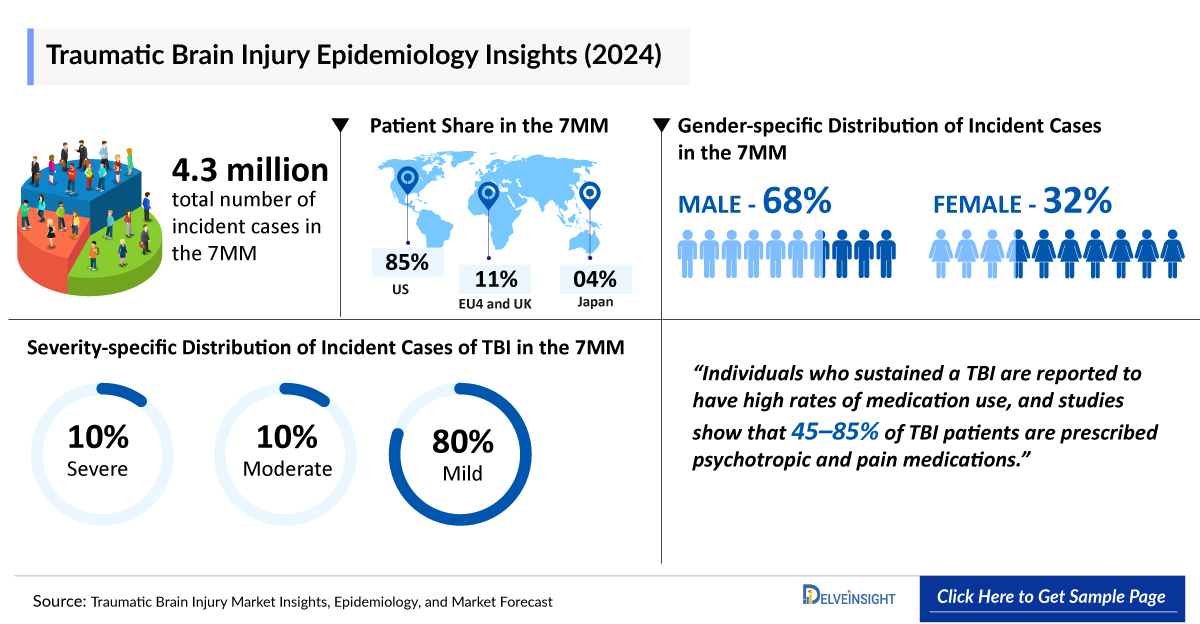
- In 2024, the seven major markets (7MM) reported nearly 4.3 million new cases of traumatic brain injury, a figure projected to rise to about 4.5 million by 2034.
- In 2024, the United States recorded approximately 3 million new cases of traumatic brain injury.
- In 2024, the severity-specific distribution of Traumatic Brain Injury in Germany, accounted for about 250,000 cases of mild Traumatic Brain Injury, about 10,500 cases of moderate Traumatic Brain Injury, and about 14,000 cases of severe Traumatic Brain Injury.Japan recorded approximately 329,000 new cases of traumatic brain injury in 2020, and this incidence is projected to grow at a CAGR of 0.01% over the forecast period.
- The highest number of incident cases was estimated in the US, followed by Japan, Germany, France, and the UK in 2024.
- Treatment for Traumatic Brain Injury varies depending on the severity of the injury. For mild cases, rest and over-the-counter pain relievers may be sufficient. Moderate-to-severe cases often require hospitalization, where treatments may include medication to reduce swelling, surgery to repair damage, and rehabilitation to regain lost skills and function. Multidisciplinary care teams tailor treatment plans to individual needs for optimal recovery.
- Currently, there are no FDA-approved treatments for acute concussion. The current standard of care is rest, followed by a gradual return to normal activity.
- Off-label therapies, including antidepressants, antiepileptics, antipsychotics, analgesics, antacids, and other symptomatic treatments, are used to manage Traumatic Brain Injury.
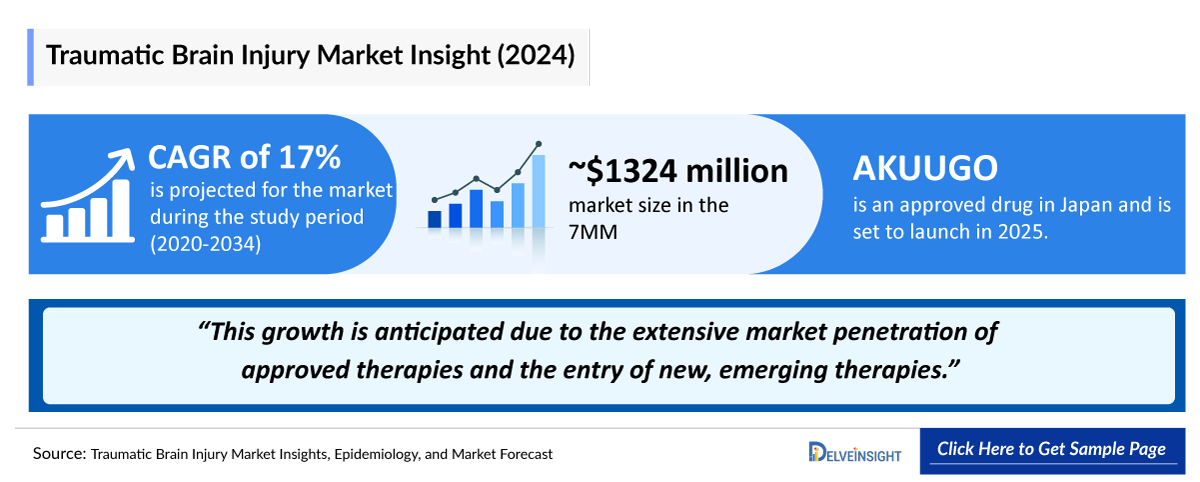
- In July 2024, the first cell therapy, AKUUGO, was approved in Japan for improving chronic motor paralysis resulting from Traumatic Brain Injury. It obtained conditional and time-limited marketing approval, which was further granted full approval in October 2025. The company is planning to launch AKUUGO in the Japanese market in 2026.
- AKUUGO has received multiple regulatory designations, including Sakigake designation (April 2019) and priority review designation (January 2022) from Japan’s MHLW, orphan regenerative medicine designation (June 2020) from MHLW, RMAT designation (September 2019) from the US FDA, and ATMP designation (April 2019) from the EMA, recognizing its potential in treating chronic Traumatic Brain Injury.
- In January 2025, SanBio entered into a manufacturing agreement with JCR Pharma for the production of AKUUGO. In addition to the stable production of commercial AKUUGO, this contract covers SanBio's future expansion of indications for cerebral infarction, etc., and the expansion of its treatment in the United States.
- There is a critical need to address both psychiatric and non-neurological symptoms in Traumatic Brain Injury care, especially in females with Traumatic Brain Injury. Additionally, reviewing the use of multiple medications is essential to mitigate the risks associated with polypharmacy.
- Companies are investigating different RoAs for Traumatic Brain Injury treatment. Oragenics, Beyond Barriers Therapeutics, and Noveome are exploring intranasal delivery, while Neuroplast is focusing on intrathecal injection as a potential approach.
- The total market size of the Traumatic Brain Injury treatment market is anticipated to grow during the forecast period due to the emergence of new and effective treatments, namely ONP-002, HB-AdMSCs, and others.
Request for unlocking the Sample Page of the "Traumatic Brain Injury Market Insights"
Key Factors Driving the Traumatic Brain Injury Market
-
Increasing Prevalence of Traumatic Brain Injuries: The rising incidence of road accidents, sports-related injuries, military trauma, and falls particularly among the aging population continues to increase the global burden of Traumatic Brain Injury, driving demand for diagnosis and treatment solutions.
-
Advancements in Diagnostic and Monitoring Technologies: Innovations in neuroimaging, blood-based biomarkers, and digital monitoring tools are enabling earlier and more accurate diagnosis of Traumatic Brain Injury, improving patient management and expanding market adoption.
-
Growing Focus on Rehabilitation and Long-Term Care: Greater emphasis on post-injury rehabilitation, including cognitive therapy and neurorehabilitation programs, is supporting sustained demand for comprehensive Traumatic Brain Injury treatment and care services.
-
Robust Research and Development of Novel Therapies: Ongoing clinical trials exploring neuroprotective drugs, stem cell therapies, and regenerative medicine approaches are strengthening the treatment pipeline and boosting market growth potential.
-
Supportive Government Initiatives and Rising Healthcare Spending: Increased investments in trauma care infrastructure, favorable reimbursement frameworks, and public health initiatives aimed at improving Traumatic Brain Injury outcomes are further propelling market expansion.
DelveInsight’s “Traumatic Brain Injury Market Insights, Epidemiology, and Market Forecast - 2034” report delivers an in-depth understanding of the Traumatic Brain Injury, historical and forecasted epidemiology and the Traumatic Brain Injury therapeutics market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
The Traumatic Brain Injury market report provides current treatment practices, emerging drugs, market share of individual therapies, and current and forecasted 7MM, Traumatic Brain Injury market size from 2020 to 2034. The report also covers current Traumatic Brain Injury treatment practices/algorithms and unmet medical needs to curate the best of the opportunities and assess the underlying potential of the market.
Scope of the Traumatic Brain Injury Market | |
|
Study Period |
2020 to 2034 |
|
Forecast Period |
2025-2034 |
|
Geographies Covered |
|
|
Traumatic Brain Injury Market |
|
|
Traumatic Brain Injury Market Size | |
|
Traumatic Brain Injury Companies |
Bristol-Myers Squibb, and others |
|
Traumatic Brain Injury Epidemiology Segmentation |
|
Traumatic Brain Injury Disease Understanding
Traumatic Brain Injury Overview
Traumatic Brain Injury is a neurological condition caused by a sudden blow, jolt, or penetrating injury to the head that disrupts normal brain function. It commonly results from incidents such as falls, road traffic accidents, sports injuries, or violence.
Traumatic Brain Injury severity ranges from mild (concussion) to moderate or severe, potentially leading to long-term cognitive, physical, emotional, and behavioral impairments. Symptoms may include headaches, memory loss, confusion, dizziness, loss of consciousness, and difficulty with speech or movement. Early diagnosis and timely management are crucial to improving outcomes and reducing long-term disability.
Traumatic Brain Injury Diagnosis
No single test is able to confirm the diagnosis of Traumatic Brain Injury definitively. Doctors assess the history of the injury, the patient’s symptoms, the physical examination, and additional tests, including neuroradiology, to confirm a diagnosis of Traumatic Brain Injury. Many Traumatic Brain Injury patients experience a loss of consciousness (blacking out) at the time of their injury. Loss of consciousness most commonly lasts from seconds to minutes, but severe Traumatic Brain Injury may last for days (coma) and may persist indefinitely in the most severe cases. Patients with mild Traumatic Brain Injury (concussion) may not experience any loss of consciousness. Most Traumatic Brain Injury patients have some degree of amnesia (loss of memory) for minutes to hours or longer surrounding their injury.
Further details related to country-based variations are provided in the report...
Traumatic Brain Injury Treatment
The management of Traumatic Brain Injury focuses on preventing primary and secondary injuries. Pre-hospital protocols ensure airway protection, oxygenation, and perfusion. Intracranial Pressure (ICP) monitoring is pivotal, with a target of 20–25 mmHg. Brain multi-modality monitoring integrates various monitors for early detection and personalized intervention. Real-time data analysis tools aid in optimizing treatment. Key monitoring probes include brain tissue oxygenation and microdialysis. These approaches, guided by evidence-based guidelines, enhance neurocritical care, mitigating ischemic risks and improving outcomes for moderate to severe Traumatic Brain Injurys.
Traumatic Brain Injury Epidemiology
As the market is derived using the patient-based model, the Traumatic Brain Injury epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by Total Incident Cases of Traumatic Brain Injury, Severity-specific Incident Cases of Traumatic Brain Injury, Gender-specific Incident Cases of Traumatic Brain Injury, and Age-specific Incident Cases of Traumatic Brain Injury in the 7MM covering the United States, EU4 countries (Germany, France, Italy, and Spain) and the United Kingdom, and Japan, from 2020 to 2034.
Key Findings in the Traumatic Brain Injury Epidemiological Analyses and Forecast
- In 2024, the 7MM had approximately 4.3 million incident cases of Traumatic Brain Injury and these cases are expected to reach 4.5 million by 2034.
- In 2024, the total incident cases of Traumatic Brain Injury were approximately 3 million in the US.
- In 2024, the severity-specific distribution of Traumatic Brain Injury in Germany, accounted for about 250,000 cases of mild Traumatic Brain Injury, about 10,500 cases of moderate Traumatic Brain Injury, and about 14,000 cases of severe Traumatic Brain Injury.
- Japan accounted for around 329,000 incident cases of Traumatic Brain Injury in 2020. These cases are expected to increase at a CAGR of 0.01% during the forecast period.
- Traumatic Brain Injury is predominantly observed in adults, accounting for approximately 71% of all cases in Italy.
- In Japan, males were more affected by Traumatic Brain Injury than females, by almost twice the ratio between them.
- In 2020, of the 2.96 million diagnosed Traumatic Brain Injurys (incidence) in the US, mortality-adjusted treated Chronic Traumatic Brain Injury patients were around 908,700.
Traumatic Brain Injury Epidemiology Segmentation
- Total Incident Cases of Traumatic Brain Injury
- Severity-specific Distribution of Incident Cases of Traumatic Brain Injury
- Gender-specific Distribution of Incident Cases of Traumatic Brain Injury
- Age-specific Distribution of Incident Cases of Traumatic Brain Injury
Traumatic Brain Injury Drug Analysis
The drug chapter segment of the Traumatic Brain Injury report encloses a detailed analysis of Traumatic Brain Injury marketed drugs and late-stage (Phase III and Phase II) Traumatic Brain Injury pipeline drugs. It also understands Traumatic Brain Injury clinical trial details, expressive pharmacological action, agreements and collaborations, approval, and patent details, advantages and disadvantages of each included drug, and the latest news and press releases.
Traumatic Brain Injury Marketed Therapy
AKUUGO (Vandefitemcel): SanBio
AKUUGO suspension for intracranial implantation is a human (allogeneic) bone marrow-derived modified mesenchymal stem cell that is produced by modifying and culturing mesenchymal stem cells derived from the bone marrow aspirate of healthy adults. It was approved in July 2024, under the Sakigake Designation Program, and obtained conditional and time-limited marketing approval for the human somatic stem cell-processed product ‘AKUUGO suspension for intracranial implantation’ in Japan for the indication of improving chronic motor paralysis resulting from Traumatic Brain Injury.
- In October 2025, SanBio announced a partial change to the marketing authorization, and a revision of the approval conditions for AKUUGO was submitted to MHLW. The proposal filed in June 2025 was accepted. Based on this review result, approval regarding the partial change by the MHLW is expected to follow. After the NHI price listing, the company plans to launch AKUUGO suspension for intracranial implantation.
- In October 2025, SanBio announced that it would advance business activities targeting Traumatic Brain Injury in the US market. They have already reached an agreement with the US FDA on the Phase III clinical trial design, and preparations for the clinical trial are scheduled to begin in the next fiscal year.
Traumatic Brain Injury Emerging Therapies
OXE103: Oxeia Biopharmaceuticals
OXE103 is synthetic human ghrelin, an endogenous hormone. OXE103 freely crosses the blood–brain barrier and helps stabilize metabolic and energy brain dysfunction following a concussion. OXE103 uniquely targets the hippocampus region of the brain, an area important for cognition and memory.
- In July 2025, Oxeia Biopharmaceuticals announced that they are looking for additional funding to start their Phase IIb clinical trial. In response to the lack of traditional investor interest, the company has engaged StartEngine, a leader in the crowdfunding sphere. OXE103’s pilot Phase IIa trial showed strong potential as an effective treatment for concussion, and they are highly encouraged and optimistic about the next Phase IIb trial.
- In February 2025, Oxeia Biopharmaceuticals announced that it is planning to initiate the Phase IIb trials for OXE103 later in 2025.
ONP-002: Oragenics
ONP-002 is a first-in-class enantiomeric neurosteroid being developed for the treatment of mTraumatic Brain Injury (concussion). The Intellectual Property (IP) encompasses the structure, synthetic preparation, and methods of use for the drug, which has demonstrated positive preclinical molecular and behavioral effects within hours in animal and cell culture models of neuronal injury.
- Oragenics remains focused on advancing ONP-002 through Phase IIa trials for concussion treatment, with trial initiation expected in Q4 2025.
- In November 2025, Oragenics announced that it is currently planning IND submission preparations for US-based Phase IIb clinical trials in Q4 2025 or Q1 2026.
- According to the company’s 10Q report in September 2025, the Phase IIb trial initiation is set for Q4 2026, and the Phase III trial start in Q4 2027.
Comparison of Traumatic Brain Injury Emerging Drugs | |||||
|
Product |
Company |
Phase |
Indications |
RoA |
Mechanism of Action |
|
OXE103 |
Oxeia Biopharmaceuticals |
II |
Concussion (mild Traumatic Brain Injury) |
SC |
Stabilize Brain Dysfunction |
|
HB-adMSCs |
Hope Biosciences |
II |
Chronic Traumatic Brain Injury |
IV infusion |
Cell replacement |
|
ONP-002 |
Oragenics |
II |
Mild Traumatic Brain Injury |
Intranasal |
Activation of the Pregnane X Receptor (PXR) |
|
MR-301 |
SHINKEI Therapeutics |
II |
Severe Traumatic Brain Injury |
IV infusion |
Inhibit dopaminergic receptor activation |
Note: Detailed emerging therapies assessment will be provided in the final report of Traumatic Brain Injury....
Traumatic Brain Injury Market Outlook
Currently, there are no approved therapies for the treatment of Traumatic Brain Injury, and only off-label treatments are used to manage its symptoms. Existing treatment options include psychostimulants, antidepressants, antiparkinsonian agents, beta-blockers, anticonvulsants, and other medications. These drugs help address the neuropsychiatric, neurocognitive, and neurobehavioral complications that arise following brain injury.
Antidepressant medications such as citalopram, methylphenidate, sertraline, and others are thought to work by affecting the levels of the brain’s natural chemical messengers, called neurotransmitters, and adjusting the brain’s response to them.
Anticonvulsant medications such as trazodone, sodium valproate, gabapentin, topiramate, and carbamazepine are used to suppress the rapid and excessive firing of neurons that start a seizure and can sometimes prevent the spread of a seizure within the brain and offer neuroprotection.
In July 2024, AKUUGO became the first and only approved brain regeneration therapy in the world. It was approved in Japan. AKUUGO was approved under the conditional and time-limited approval. Allogeneic cell therapy has been proven effective against chronic motor paralysis caused by Traumatic Brain Injury. The company is planning to actively promote its use in various central nervous system diseases that have unmet medical needs, in addition to this indication. With a partial change in the approval conditions for AKUUGO, it has now received full regulatory access, and the plans for market launch are underway with NHI pricing decisions expected soon. Discussions with the US FDA for the Phase III trial have been concluded, and the trial is expected to start by 2026.
- The market for Traumatic Brain Injury is expected to experience positive growth with the approval of potential drugs like OXE103, ONP-002, MR-301, and others.
- The total market size of Traumatic Brain Injury in the 7MM was around USD 1,300 million in 2024. This is estimated to increase by 2034.
- Among the 7MM, the US captured the highest market in 2024, covering a total of 85% market, followed by Japan, which is anticipated to grow during the forecast period (2025–2034).
- In 2024, EU4 and the UK, Germany captured nearly 29% of the total market, followed by France (22%) in the 7MM.
- AKUUGO’s anticipated Japanese market entry in 2026 is poised to significantly reshape the Traumatic Brain Injury treatment landscape. With a planned Phase III trial in the US, SanBio is positioning itself to secure a strong global market presence. The company’s strategic clinical and commercial development efforts suggest substantial future impact as the demand for effective Traumatic Brain Injury therapies continues to grow.
Traumatic Brain Injury Competitive Landscape
The competitive landscape of the Traumatic Brain Injury (Traumatic Brain Injury) market is shaped by a mix of established healthcare firms, specialized medical device manufacturers, and emerging biotech companies. Key players are focusing on innovation, strategic partnerships, and product pipeline expansion to gain market share. Competition centers on the development of advanced diagnostic tools, such as high-resolution imaging systems and biomarker assays, as well as novel therapeutic solutions aimed at neuroprotection and enhanced recovery. Companies are also investing in clinical research and collaborations with academic institutions to strengthen their Traumatic Brain Injury portfolios.
Additionally, mergers and acquisitions are common as larger firms seek to broaden their offerings and accelerate entry into new geographic markets. Overall, the competitive landscape is dynamic, driven by technological advancements and the growing demand for improved patient outcomes.
Key Traumatic Brain Injury Companies
The Key Traumatic Brain Injury companies actively involved in the Traumatic Brain Injury treatment landscape include -
- Oxeia Biopharmaceuticals
- Hope Biosciences
- Oragenics
- SHINKEI Therapeutics
- BioVie
- Beyond Barriers Therapeutics
- AivoCode
- Abliva/Owl Therapeutics
- Neuroplast and others
Traumatic Brain Injury Drug Class Insight
Several promising therapies are currently under development for neurological conditions, including traumatic brain injury (Traumatic Brain Injury), each representing diverse drug classes. AKUUGO (Vandefitemcel) is a therapy based on human (allogeneic) bone marrow-derived modified mesenchymal stem cells, offering regenerative potential. OXE103, a synthetic human ghrelin, aims to modulate neuroprotective pathways and support brain recovery. HB-adMSCs, derived from adipose mesenchymal stem cells, represent another stem cell-based approach with the ability to reduce inflammation and promote healing. ONP-002, a neurosteroid, is being investigated for its capacity to stabilize neural function and mitigate damage following Traumatic Brain Injury. Lastly, MR-301 (Amantadine HCl IV Solution) is a small molecule therapy designed to enhance arousal and cognitive function. Together, these innovative treatments highlight the growing diversity and sophistication of the therapeutic landscape for Traumatic Brain Injury and related conditions.
Cell Replacements
Cell replacement therapies for Traumatic Brain Injury primarily act through mechanisms like creating structural "biobridges," modulating inflammation, and enhancing endogenous repair. Modified mesenchymal stromal cells (e.g., SB623) establish neurovascular pathways between neurogenic niches (e.g., subventricular zone) and injury sites, enabling migration of host stem cells via Matrix Metalloproteinase-9 (MMP-9)-rich signaling. These transplanted cells secrete growth factors (e.g., Brain-derived neurotrophic factor [BDNF], Nerve growth factor [NGF]) and anti-inflammatory cytokines, reducing neuronal apoptosis, promoting angiogenesis, and improving synaptic plasticity. Additionally, stem cell-derived exosomes attenuate neuroinflammation and enhance motor recovery.
Traumatic Brain Injury Treatment Landscape
Traumatic Brain Injury is a complex condition caused by an external impact to the head, leading to brain damage. Managing Traumatic Brain Injury is challenging yet essential, requiring medical intervention, rehabilitation, and symptom management. Treatment approaches range from cognitive therapy to radical surgeries like bilateral decompressive craniotomies. While guidelines exist, they must be adapted to individual cases due to healthcare constraints.
Currently, no FDA-approved therapies for Traumatic Brain Injury exist, and only off-label medications are used to manage its symptoms. Available treatments include psychostimulants, antidepressants, anticonvulsants, beta-blockers, and pain relievers, addressing neuropsychiatric, neurocognitive, and motor complications. Advances in acute care have led to a growing population of Traumatic Brain Injury survivors, many with long-term disabilities, highlighting the urgent need for approved treatment options.
Pharmaceutical companies have made significant investments in Traumatic Brain Injury drug development, although the late-stage pipeline remains limited. However, several early-phase clinical trials are ongoing, with promising mid- and late-stage trial results expected to expand the Traumatic Brain Injury therapeutic market in the coming years. The drug pipeline primarily targets acute Traumatic Brain Injury, with key players such as Oxeia Biopharmaceuticals (OXE103), Hope Biosciences (HB-adMSCs), Oragenics (ONP-002), SHINKEI Therapeutics (MR-301 [Amantadine HCl IV Solution]), and Cellvation/Fortress Biotech (CEVA101) actively developing potential therapies. Despite challenges, ongoing research and investment offer hope for future breakthroughs in Traumatic Brain Injury treatment, paving the way for improved care and recovery options.
Traumatic Brain Injury Drugs Uptake
This section focuses on the uptake rate of potential drugs expected to launch in the market during 2025–2034. For example, ONP-002 in the US is expected to be launched by 2030 in the US.
Further detailed analysis of emerging therapies, drug uptake in the report…
Traumatic Brain Injury Clinical Trial Activities
The Traumatic Brain Injury pipeline report provides insights into different Traumatic Brain Injury clinical trials within Phase III, Phase II, and Phase I stages. It also analyzes key players involved in developing targeted therapeutics.
Traumatic Brain Injury Pipeline Development Activities
The Traumatic Brain Injury clinical trial analysis report covers information on collaborations, acquisitions and mergers, licensing, and patent details for Traumatic Brain Injury emerging therapies.
|
Region |
KOL Views |
|
United States |
“Concussion has been used synonymously with the term mild Traumatic Brain Injury. Emerging classification mechanisms specific to concussions promote the separation of these terms. Mild injuries likely account for more than 80% of all Traumatic Brain Injurys, although the true incidence is difficult to ascertain because many patients who sustain these injuries do not seek medical attention, and therefore, they are not documented or tracked.” – MD, McGovern Medical School, US |
|
United Kingdom |
“Traumatic Brain Injury is a major global health challenge and priority. There are several promising lines of research directed towards optimizing neurointensive care protocols, developing an evidence base for surgical intervention, and translating promising neuroprotective pharmacotherapies into application to human pathology. The importance of personalized medicine and combinatorial therapy in the field of Traumatic Brain Injury cannot be overstated, given the heterogeneity of the pathology such that a single intervention cannot address every pathological mechanism at play.” – Associate Professor, University of Cambridge, UK |
Latest KOL Views on Traumatic Brain Injury Market Report
To keep up with current market trends, we take KOLs and SMEs’ opinions working in the domain through primary research to fill the data gaps and validate the secondary research. Industry Experts were contacted for insights on Traumatic Brain Injury evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake along with challenges related to accessibility, including KOL from McGovern Medical School, US; University of Cambridge, UK; University of Florida, US; Harvard Medical School, US; Department of Traumatology and Acute Critical Medicine, Japan; BioCruces Bizkaia Health Research Institute, Spain; and others.
DelveInsight’s analysts connected with 50+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. Their opinion helps to understand and validate current and emerging therapies and treatment patterns or Traumatic Brain Injury market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
Traumatic Brain Injury Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Conjoint Analysis analyzes multiple approved and emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the route of administration, order of entry and designation, probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Traumatic Brain Injury Market Access and Reimbursement
The high cost of therapies for treatment is a major factor restraining the growth of the drug market. Because of the high cost, the economic burden is increasing, leading the patient to escape from proper treatment.
The report further provides detailed insights on the country accessibility and reimbursement scenarios, cost-effectiveness scenario of approved therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Scope of the Traumatic Brain Injury Market Report
- The report covers a segment of key events, an executive summary, a descriptive overview of Traumatic Brain Injury, explaining its causes, signs, and symptoms, and currently available therapies.
- Comprehensive insight has been provided into the epidemiology segments and forecasts, the future growth potential of the diagnosis rate, disease progression, and treatment guidelines.
- Additionally, an all-inclusive account of the current and emerging therapies, along with the elaborative profiles of late-stage and prominent therapies, will affect the current treatment landscape.
- A detailed review of the Traumatic Brain Injury market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind the approach is included in the report covering the 7MM drug outreach.
- The report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preference that help shape and drive the 7MM Traumatic Brain Injury market.
Traumatic Brain Injury Market Report Insights
- Traumatic Brain Injury Patient Population
- Traumatic Brain Injury Therapeutic Approaches
- Traumatic Brain Injury Pipeline Analysis
- Traumatic Brain Injury Market Size and Trends
- Existing and Future Market Opportunity
Traumatic Brain Injury Market Report Key Strengths
- 10 years Forecast
- The 7MM Coverage
- Traumatic Brain Injury Epidemiology Segmentation
- Key Cross Competition
- Conjoint Analysis
- Traumatic Brain Injury Drugs Uptake
- Key Market Forecast Assumptions
Traumatic Brain Injury Market Report Assessment
- Current Traumatic Brain Injury Treatment Practices
- Traumatic Brain Injury Unmet Needs
- Traumatic Brain Injury Pipeline Product Profiles
- Traumatic Brain Injury Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
- Traumatic Brain Injury Market Drivers
- Traumatic Brain Injury Market Barriers
FAQ Related to the Traumatic Brain Injury Market Report:
- What was the Traumatic Brain Injury market share (%) distribution in 2020, and what will it look like in 2034?
- At what CAGR is the Traumatic Brain Injury market expected to grow at the 7MM level during the study period (2020–2034)?
- What are the disease risks, burdens, and unmet needs of Traumatic Brain Injury?
- What is the historical Traumatic Brain Injury patient pool in the United States, the EU4 (Germany, France, Italy, and Spain), the UK, and Japan?
- What will be the growth opportunities across the 7MM concerning the patient population of Traumatic Brain Injury?
- At what CAGR is the population expected to grow across the 7MM during the study period (2020–2034)?
- How many companies are developing therapies for the treatment of Traumatic Brain Injury?
- What are the key collaborations (Industry–Industry, Industry-Academia), Mergers and acquisitions, and licensing activities related to Traumatic Brain Injury?
- What are the recent novel therapies, targets, mechanisms of action, and technologies developed to overcome the limitations of existing therapies?
- What are the key designations that have been granted for the emerging therapies for Traumatic Brain Injury?
Reasons to Buy Traumatic Brain Injury Market Forecast Report
- The report will help develop business strategies by understanding the latest trends and changing treatment dynamics driving the Traumatic Brain Injury Market.
- Insights on patient burden/disease incidence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- To understand the existing market opportunity in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identifying strong upcoming players in the market will help devise strategies that will help get ahead of competitors.
- Detailed analysis, ranking of class-wise potential current, and emerging therapies under the conjoint analysis section to provide visibility around leading classes.
- Highlights of Access and Reimbursement policies of approved therapies, barriers to accessibility of off-label expensive therapies, and patient assistance programs.
- To understand the perspective of Key Opinion Leaders around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights into the unmet needs of the existing market so that the upcoming players can strengthen their development and launch strategy.


-pipeline.png&w=256&q=75)



