Triple Negative Breast Cancer Market Summary
- The Triple Negative Breast Cancer Market in the 7MM is expected to grow from USD 4,676 million in 2025 to USD 7,083 million in 2034.
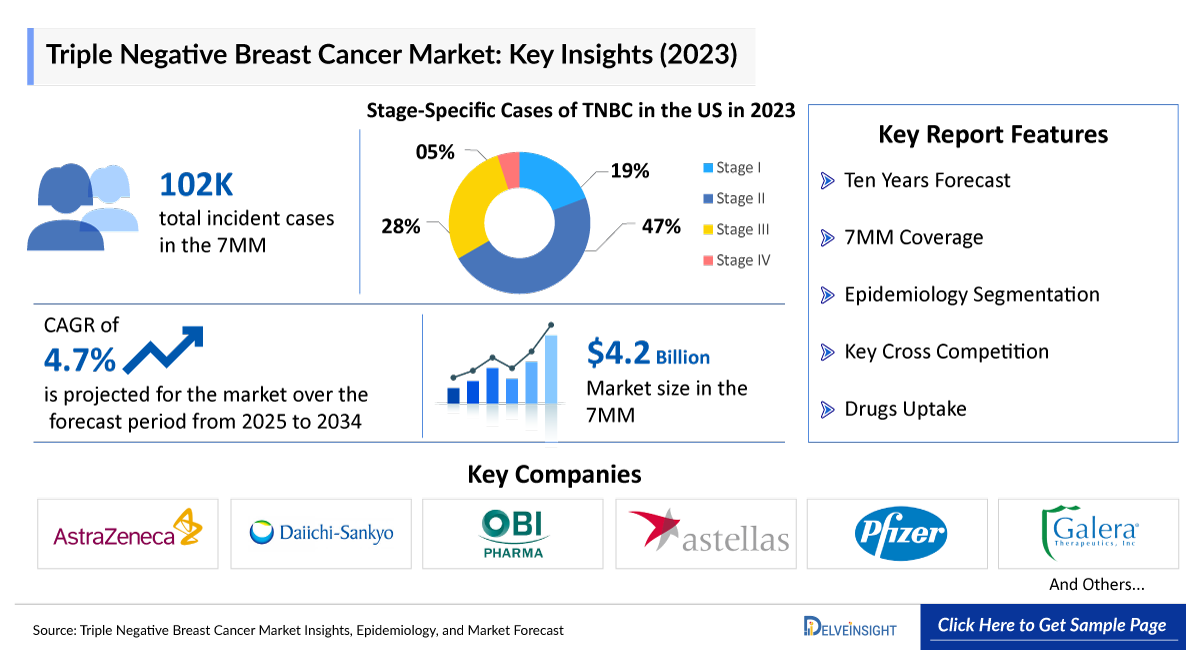
- The Triple Negative Breast Cancer Market is projected to grow at a CAGR of 4.70% by 2034 in leading countries like the US, EU4, UK, and Japan.
Triple Negative Breast Cancer Market and Epidemiology Analysis
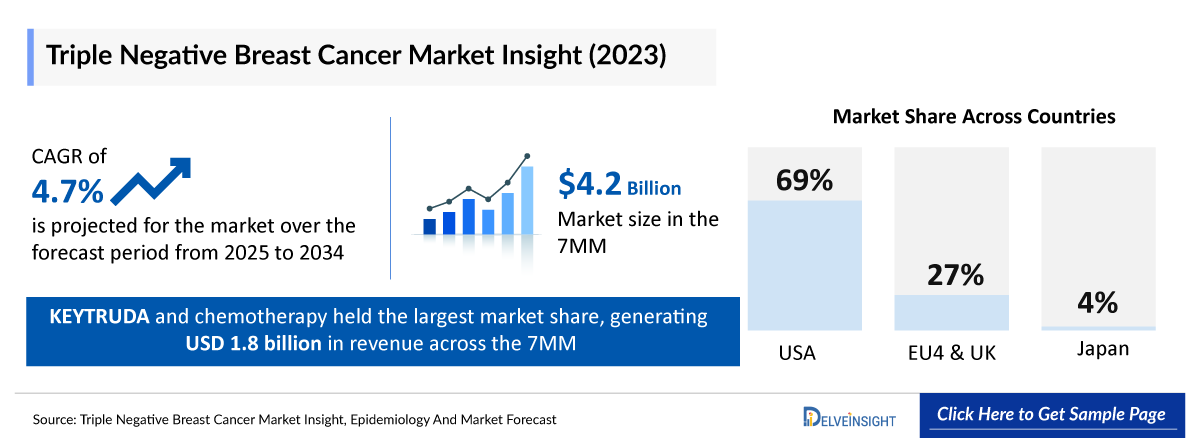
- According to DelveInsight’s analysis, the TNBC market in the 7MM was valued at approximately USD 4,200 million in 2023. Over the forecast period from 2025 to 2034, Triple Negative Breast Cancer market is projected to grow at a CAGR of 4.7%.
- The TNBC market is poised for steady growth, with a strong compound annual growth rate (CAGR) projected from 2025 to 2034. This expansion across the 7MM will be driven by the launch of innovative therapies, including DATROWAY, DATROWAY in combination with IMFINZI, Adagloxad simolenin, Sacituzumab govitecan-hziy in combination with pembrolizumab, PADCEV, Tilarginine, IMFINZI (durvalumab) with paclitaxel and BNT327/ PM8002 in combination with Chemotherapy.
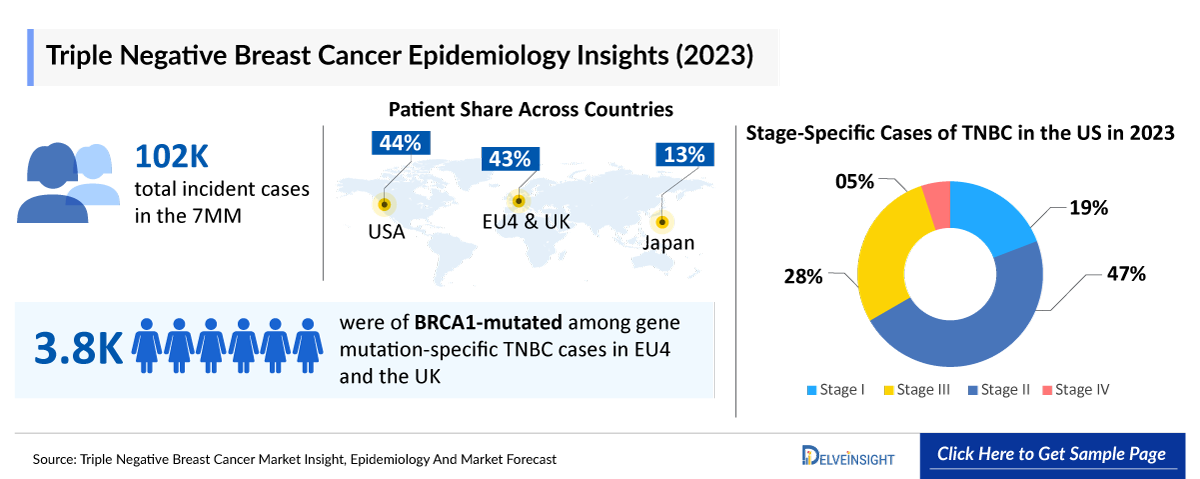
- According to DelveInsight’s estimates, in 2023, there were approximately 102 thousand incident cases of TNBC in the 7MM. Of these, the United States accounted for 44% of the cases, while EU4 and the UK accounted for nearly 43% and Japan represented 13% of the cases, respectively.
- Merck, Roche, AstraZeneca, Pfizer and Gilead Sciences have been the leading players in the Triple Negative Breast Cancer market, offering treatments like KEYTRUDA, TECENTRIQ, LYNPARZA, TALZENNA and TRODELVY.
- The absence of a definitive treatment drives continuous research, paving the way for personalized therapies and innovative approaches that offer more effective and tailored solutions for TNBC.
- Despite early diagnosis, TNBC remains prone to recurrence, underscoring the limitations of current therapies in achieving sustained remission. Advancing more durable and effective treatment approaches is essential to improving long-term outcomes and reducing the risk of disease progression.
Triple Negative Breast cancer (TNBC) Market Size and Forecast
- 2025 Triple Negative Breast cancer (TNBC) Market Size: 4,676 Million in 2025
- 2034 Projected Triple Negative Breast cancer (TNBC) Market Size: 7,083 Million in 2034
- Growth Rate (2025-2034): 4.70%% CAGR
- Largest Triple Negative Breast cancer (TNBC) Market: United States
DelveInsight’s “Triple Negative Breast Cancer (TNBC) Market Insights, Epidemiology, and Market Forecast 2034” report delivers an in-depth understanding of TNBC, historical and forecasted epidemiology, as well as the TNBC market trends in the United States, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
The TNBC market report provides current treatment practices, emerging drugs, market share of individual therapies, and current and forecasted 7MM TNBC market size from 2020 to 2034. The report also covers TNBC treatment practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s potential.
Key Factors Driving the Growth of the Triple-negative Breast Cancer Market
Rising TNBC Incidence & Prevalence
TNBC accounts for about 15–20% of all breast cancer cases worldwide. In the US, the incident cases are expected to rise at a CAGR of 1% by 2034.
Advances in Targeted & Immunotherapies
The emergence of checkpoint inhibitors (e.g., anti–PD-1/PD-L1), PARP inhibitors, ADCs, and other precision therapies is expanding treatment options and improving outcomes.
High Unmet Need in TNBC Market
TNBC lacks expression of ER, PR, and HER2, making it unresponsive to conventional targeted and hormonal therapies. This creates a significant demand for novel treatment options.
Rising TNBC Competitive Landscape
There is a significant need for new treatments for TNBC, as current therapies often fail to manage the disease entirely. Innovative drug development could offer more effective options, improving outcomes for patients with this aggressive cancer. Several promising TNBC drugs are currently in clinical trials, including DATROWAY (AstraZeneca/Daiichi Sankyo), Adagloxad Simolenin (OBI Pharma), PADCEV (Astellas Pharma/Pfizer), BNT327/PM8002 (BioNTech), and Tilarginine (Galera Therapeutics), among others.
Triple Negative Breast Cancer Disease Understanding
Triple Negative Breast Cancer (TNBC) overview
TNBC is a highly aggressive and heterogeneous subtype of breast cancer characterized by the absence of estrogen receptors (ER), progesterone receptors (PR), and human epidermal growth factor receptor 2 (HER2). It represents a significant portion of breast cancer cases and is known for its rapid progression, high recurrence rates, and poor prognosis. TNBC is more commonly diagnosed in younger women, African American populations, and those with BRCA1 gene mutations.
The biological complexity of TNBC is reflected in its distinct immune microenvironment, which includes elevated levels of vascular endothelial growth factors, tumor-infiltrating lymphocytes (TILs), and tumor-associated macrophages (TAMs). These factors contribute to tumor growth, invasion, and metastasis. TNBC has a higher likelihood of distant metastasis, particularly to the lungs, liver, and brain, within a shorter time frame compared to other breast cancer subtypes.
Risk factors for TNBC are categorized as modifiable and non-modifiable. Non-modifiable factors include age, sex, genetic predisposition, family history, and breast tissue density, while modifiable factors involve obesity, chemical exposures, and certain drug use. Given its aggressive nature and lack of hormone receptor expression, TNBC presents significant challenges in disease management, necessitating ongoing research to improve patient outcomes.
Triple Negative Breast Cancer (TNBC) diagnosis
The diagnosis of TNBC involves imaging techniques such as mammography, ultrasound, and MRI, followed by biopsy methods like core biopsy, fine needle aspiration, and surgical biopsy. TNBC is typically high-grade, with poorly differentiated cells. Staging follows the TNM system, assessing tumor size, lymph node involvement, and metastasis to determine disease severity.
Further details related to country-based variations are provided in the report…
Triple Negative Breast Cancer (TNBC) treatment
TNBC treatment includes standard options like surgery, chemotherapy, radiation, and immunotherapy. Surgery typically involves lumpectomy or mastectomy with lymph node evaluation. Radiation targets residual cancer cells, while chemotherapy remains the primary systemic treatment. Immunotherapy, particularly PD-1/PD-L1 inhibitors, enhances immune response and is being explored for advanced and high-risk TNBC cases.
Triple Negative Breast Cancer (TNBC) Epidemiology
As the market is derived using a patient-based model, the TNBC epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by Total Incident Cases of Breast Cancer, Total Incident Cases of TNBC, Gene Mutation-specific Incident Cases of TNBC, Stage-specific Incident Cases of TNBC, Age-specific Incident Cases of TNBC and Line-wise Treated Incident Cases of TNBC in the 7MM covering the United States, EU4 countries (Germany, France, Italy, and Spain), the United Kingdom, and Japan from 2020 to 2034.
Key findings from the Triple Negative Breast Cancer Epidemiological Analysis and Forecasts:
- According to DelveInsight's estimates, the total incident cases of breast cancer in the 7MM were nearly 680 thousand in 2023.
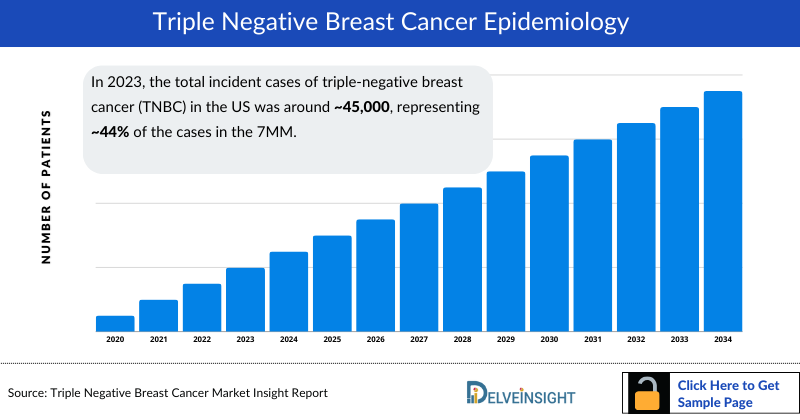
- In 2023, the US accounted for the highest incidence of triple negative breast cancer with approximately 45,000 cases, which are expected to increase by 2034 at a CAGR of 1% over the forecast period from 2025 to 2034.
- In 2023, the Germany reported the highest incidence of triple negative breast cancer among the EU4 and the UK, with approximately 11,000 cases. France followed with nearly 10,000 cases, while Spain recorded the lowest triple negative breast cancer incidence, with nearly 5,000 cases.
- In Japan, Stage II TNBC had the highest number of cases in 2023, totaling 6,000. Additionally, there were nearly 3,000 cases in Stage I, 4,000 in Stage III, and 700 in Stage IV.
- In EU4 and the UK, BRCA1-mutated TNBC had the highest number of cases among gene mutation-specific TNBC cases in 2023, around 3,800 cases.
- In France, the highest number of cases was recorded in individuals below 45 years of age, totaling 3,200 cases in 2023.
- In 2023, Spain reported nearly 5,000 incident cases of TNBC and this figure is expected to increase by 2034 during the forecast period (2025-2034).
- In Italy, Stage II TNBC had the highest number of cases in 2023, around 4,000. Additionally, there were nearly 1,700 cases in Stage I, 2,500 in Stage III, and 500 in Stage IV.
- In 2023, Germany reported approximately 8,000 cases in Neoadjuvant/adjuvant, 5,300 cases in the 1L, 2,800 in the 2L, and 1,700 in the 3L and above treatment. This trend is expected to increase by 2034, reflecting a distribution in treatment pattern over time.
Triple Negative Breast Cancer Epidemiology Segmentation:
- Total Incident Cases of Breast Cancer
- Total Incident Cases of TNBC
- Gene Mutation-specific Incident Cases of TNBC
- Stage-specific Incident Cases of TNBC
- Age-specific Incident Cases of TNBC
- Line-wise Treated Incident Cases of TNBC
Triple Negative Breast Cancer Market Recent Developments and Breakthroughs:
- In May 2025, UTR Therapeutics Inc. announced the submission of an IND application to the FDA for UTRxM1-18, a novel therapy designed to target c-MYC driven cancers, including triple-negative breast, pancreatic, colorectal, and ovarian cancers. Leveraging its 3’UTR engineering platform, UTRxM1-18 selectively degrades cancer-specific transcripts while sparing healthy cells. In preclinical studies, the drug showed strong, dose-dependent efficacy across tumor types with no dose-limiting toxicities.
- In May 2025, Lantern Pharma Inc. announced that it has received clearance from the U.S. Food and Drug Administration (FDA) for its Investigational New Drug Application (IND) to conduct a Phase 1b/2 clinical trial for LP-184 in Triple Negative Breast Cancer. This milestone follows previous regulatory achievements, including Orphan Drug Designation in 2023 and Fast Track Designation in 2024.
- In April 2025, Gilead Sciences reported positive results from the Phase 3 ASCENT-04/KEYNOTE-D19 study, showing that the combination of Trodelvy® (sacituzumab govitecan-hziy) and Keytruda® (pembrolizumab) significantly improved progression-free survival in patients with metastatic triple-negative breast cancer (mTNBC) whose tumors express PD-L1 (CPS ≥ 10), compared to chemotherapy and Keytruda alone.
Triple Negative Breast Cancer Drug Chapter Analysis
The drug chapter segment of the TNBC market report encloses a detailed analysis of TNBC marketed drugs and mid to late-stage (Phase III and Phase II) pipeline drugs. It also helps understand the TNBC clinical trial details, expressive pharmacological action, agreements and collaborations and approval, advantages and disadvantages of each included drug, and the latest news and press releases.
Triple Negative Breast Cancer Marketed Drugs
KEYTRUDA (Pembrolizumab): Merck
KEYTRUDA (pembrolizumab) is an immunotherapy that strengthens the immune system's ability to target and destroy cancer cells. As a humanized monoclonal antibody, it blocks the PD-1 receptor, preventing its interaction with PD-L1 and PD-L2 ligands, which in turn activates T cells to attack both cancerous and healthy tissues. In TNBC, KEYTRUDA has shown efficacy in combination with chemotherapy. The KEYNOTE-355 trial assessed its use with chemotherapy agents like paclitaxel and gemcitabine in metastatic cases, while KEYNOTE-522 explored its potential in early-stage TNBC. Approved in the US, EU4 and the UK, and Japan, KEYTRUDA holds Priority Review and Breakthrough Designation (BTD).
TRODELVY (sacitzumab govitecan-hziy): Gilead Sciences
TRODELVY (sacituzumab govitecan-hziy), an ADC targeting TROP-2, is approved for metastatic TNBC (mTNBC) patients who have received prior treatment. It delivers a topoisomerase I inhibitor directly to cancer cells, improving efficacy while reducing harm to healthy tissues. Approved in the US, EU4 and the UK, and Japan, it holds Priority Review, BTD, and Fast Track Designation (FTD). Supported by the Phase III ASCENT trial, it is also being studied with pembrolizumab in ongoing trials. The US patent expires in 2028, and the EU patent in 2029.
TALZENNA (talazoparib): Pfizer
Talazoparib is a small-molecule PARP inhibitor that disrupts DNA repair in cancer cells by inhibiting PARP enzymes, leading to decreased tumor growth and enhanced cancer cell death. Proven effective in both BRCA1/2-mutated and wild-type breast cancers, it was approved based on the EMBRACA trial involving 431 patients with gBRCAm HER2-negative metastatic or locally advanced breast cancer. Talazoparib is administered orally and targets second-line or later therapies. It is approved in the US, EU4 and the UK, and Japan, with Priority Review Designation, and its patents are set to expire in 2029 in the US and Japan, and 2034 in Europe.
|
MoA |
RoA |
|
PD-1 inhibitors |
IV |
|
IV | |
|
PARP inhibitor |
Oral |
Triple Negative Breast Cancer Emerging Drugs
DATROWAY (Datopotamab Deruxtecan): AstraZeneca/Daiichi Sankyo
Datopotamab Deruxtecan (Dato-DXd), or DATROWAY, is a precision medicine and investigational antibody drug conjugate (ADC) for TNBC, being evaluated as monotherapy and with IMFINZI in neoadjuvant, adjuvant, and metastatic settings. With Phase III data expected by 2026, it holds promise for advancing TNBC treatment across various disease stages.
In December 2023, two Phase III clinical trials evaluating the combination of datopotamab deruxtecan and durvalumab were launched to investigate their effectiveness in patients with two distinct subtypes of breast cancer.
Adagloxad Simolenin: OBI Pharma
Adagloxad simolenin, a therapeutic cancer vaccine, targets the tumor-associated glycan antigen Globo H. This subcutaneous (SC) immunotherapy, a conjugate of Globo H and KLH, stimulates antibody production against cancer cells. Licensed by OBI from Merck, clinical trials at Memorial Sloan Kettering Cancer Center (MSKCC) confirmed its safety and strong immune response in metastatic breast cancer.
Currently, it is being investigated in Phase III in patient with high risk, early stage Globo H+ TNBC.
In January 2024, DSMB positively responded to the first interim analysis of the Phase III clinical trials of Adagloxad Simolenin (OBI-822)/OBI-821 active immunity vaccine for TNBC and suggested the company to continue the trials.
PADCEV (Enfortumab vedotin): Astellas Pharma/Pfizer
Enfortumab vedotin-ejfv is an ADC designed for targeted cancer therapy, specifically binding to Nectin-4, a protein overexpressed in tumors. This monoclonal antibody is linked to Monomethyl Auristatin E (MMAE), a microtubule-disrupting agent. Administered via IV infusion, it induces apoptosis in cancer cells. Currently, it is being evaluated in combination with pembrolizumab for TNBC and other solid tumors in 2L+ therapy, offering hope for treatment-resistant cancers.
Currently, it is being investigated in Phase II in patients with locally advanced or metastatic malignant solid tumors in EV-202.
|
MoA |
RoA |
Company |
|
TROP2-directed DXd ADC |
IV infusion |
AstraZeneca/Daiichi Sankyo |
|
Anti-tumor surface glycan antigen Globo H |
SC injection |
OBI Pharma |
|
Anti Nectin-4 protein |
IV infusion |
Astellas Pharma/ Pfizer |
TNBC Drug Class Analysis
The treatment of TNBC primarily involves neoadjuvant or adjuvant therapy as an initial approach, often followed by first-line treatment for advanced cases. If the disease progresses or does not respond adequately, second-line and subsequent therapies are considered. Immunotherapy, such as PD-1/PD-L1 inhibitors, may also be utilized in metastatic settings to enhance the immune response against the tumor.
Emerging Triple Negative Breast Cancer therapies include DATROWAY (Datopotamab Deruxtecan), Adagloxad Simolenin, and Tilarginine
Adagloxad Simolenin, developed by OBI Pharma, targets the tumor-associated glycan antigen Globo H. Administered via SC injection, it is currently in Phase III Triple Negative Breast Cancer clinical trials.
Tilarginine, developed by Galera Therapeutics, is a Nitric Oxide Synthase (NOS) inhibitor administered via IV infusion. It is currently in Phase II clinical trials for the treatment of TNBC.
Continued in report…
TNBC Market Outlook
TNBC lacks hormone receptors and HER2 expression, making it unresponsive to hormonal or HER2-targeted therapies. As a result, systemic chemotherapy has traditionally been the primary treatment, particularly for metastatic cases. The treatment approach typically begins with neoadjuvant or adjuvant therapy, followed by first-line treatment for advanced cases. If the disease progresses or does not respond adequately, second-line and subsequent therapies are considered. Immunotherapy, such as PD-1/PD-L1 inhibitors, is increasingly used in metastatic settings to enhance the immune response against the tumor.
For early-stage TNBC, KEYTRUDA (Pembrolizumab), a PD-1 inhibitor, is now a standard treatment, combined with neoadjuvant chemotherapy and continued as adjuvant monotherapy. It is also used with chemotherapy for locally recurrent or metastatic TNBC in PD-L1-positive patients (CPS =10). Targeted therapies have also emerged, particularly for BRCA-mutated TNBC, where PARP inhibitors like LYNPARZA (Olaparib) and TALZENNA (Talazoparib) exploit impaired DNA repair mechanisms. TRODELVY (Sacituzumab govitecan), a TROP-2-directed ADC, is used in recurrent or refractory metastatic TNBC. While TECENTRIQ (Atezolizumab) was withdrawn in the US due to failed confirmatory trials, it remains available in Europe and Japan.
Taxane-based chemotherapies remain the standard treatment option, often combined with immune checkpoint inhibitors for PD-L1-positive cases. Platinum-based chemotherapies, though sometimes used, are debated due to severe side effects and limited efficacy. Despite advancements, TNBC remains aggressive, necessitating ongoing research to improve treatment options and survival rates.
There is a significant need for new treatments for TNBC, as current therapies often fail to fully manage the disease. Innovative drug development could offer more effective options, improving outcomes for patients with this aggressive cancer. Several promising Triple Negative Breast Cancer drugs are currently in the pipeline, including DATROWAY (Datopotamab Deruxtecan), Adagloxad Simolenin, PADCEV (Enfortumab vedotin), BNT327/PM8002, and Tilarginine, among others.
- In 2023, the TNBC market size in the US was around USD 2,900 million, accounting for 69% of the total Triple negative breast cancer market. This figure is expected to grow significantly with the introduction of emerging therapies.
- The total Triple Negative Breast Cancer market size of EU4 and the UK was estimated to be approximately USD 1,100 million in 2023, which was nearly 27% of the total Triple Negative Breast Cancer market revenue for the 7MM.
- Among EU4 and the UK, Germany accounted for the highest Triple Negative Breast Cancer market share with approximately USD 300 million in 2023, followed by France with approximately USD 250 million. Spain accounted for the smallest TNBC market share, with nearly USD 130 million in the same year.
- In 2023, the total Triple Negative Breast Cancer market size was approximately USD 170 million in Japan which is anticipated to increase during the forecast period (2025–2034).
- As per the estimates, among the current treatment options, KEYTRUDA (pembrolizumab) and chemotherapy held the largest Triple Negative Breast Cancer treatment market share, generating approximately USD 1,800 million in revenue in 2023 across the 7MM.
Triple Negative Breast Cancer (TNBC) Drugs Uptake
This section focuses on the uptake rate of potential TNBC drugs expected to be launched in the Triple Negative Breast Cancer drugs market during 2020–2034.
Further detailed analysis of emerging therapies drug uptake in the report…
Triple Negative Breast Cancer (TNBC) Pipeline Development Activities
The Triple Negative Breast Cancer Treatment market report provides insights into different therapeutic candidates in Phase III, Phase II, and Phase I. It also analyzes key TNBC companies involved in developing targeted therapeutics.
Pipeline development activities
The TNBC Treatment market report covers information on collaborations, acquisitions and mergers, licensing, and patent details for emerging therapies for TNBC.
Latest KOL Views
To keep up with current Triple Negative Breast Cancer market trends, we take KOLs and SMEs’ opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts contacted for insights on TNBC evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake, along with challenges related to accessibility, including Medical/scientific writers, Medical Professionals, Professors, Directors, and Others.
DelveInsight’s analysts connected with 30+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. Centers like the Icahn School of Medicine, USA, Dana-Farber Cancer Institute, USA, NewYork-Presbyterian / Columbia University Irving Medical Center, USA, University of Tübingen, Germany, Institut Gustave Roussy, France, University of Messina, Italy, Hospital Universitario de Torrejon, Spain, University of Nottingham, the UK, Fukushima Medical University, Japan, St. Luke’s International Hospital, Japan among others, were contacted. Their opinion helps understand and validate current and emerging therapy treatment patterns or TNBC market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the Triple Negative Breast Cancer therapeutics market and the unmet needs.
Physician’s View
As per the KOLs from the US, “TNBC presents a significant treatment challenge due to the limited options beyond chemotherapy. Unlike other breast cancer subtypes, the availability of targeted therapies remains scarce. A key concern is the incomplete response of many triple-negative tumors to even the most effective chemotherapy regimens, with cancer stem cells playing a crucial role in driving this resistance. The underlying mechanisms behind this phenomenon remain largely unexplored, highlighting the need for further research to enhance therapeutic strategies”.
As per the KOLs from Germany, “TNBC should be classified based on histological morphology, with special subtypes identified and supported by genetic profiling where feasible. A thorough histopathological evaluation is essential to differentiate pure from mixed forms and to distinguish low-grade tumors from high-grade variants with distinct clinical behavior. In challenging cases, seeking a second expert opinion is crucial for accurate diagnosis and optimal patient management. Not all TNBC patients necessarily require systemic chemotherapy, emphasizing the need for case-by-case assessment in Tumor Board or Multidisciplinary Team discussions. Clinical studies and trials should incorporate histological subtyping to ensure appropriate cohort stratification”.
As per the KOLs from Japan, “TNBC typically exhibits an expanding growth pattern with minimal intraductal spread, making it a strong candidate for Breast-conserving Therapy (BCT) when adequate margins are achieved. While the local recurrence rate after BCT remains relatively low compared to other subtypes, regional recurrence is notably higher, necessitating meticulous sentinel node biopsy and axillary resection. Radiation therapy plays a crucial role in TNBC management, with post-mastectomy chest wall and regional irradiation, as well as radiation following BCT, being key considerations for optimal outcomes.”
Qualitative Analysis
We perform Qualitative and Triple Negative Breast Cancer market Intelligence analysis using various approaches, such as SWOT and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Conjoint Analysis analyzes multiple emerging Triple Negative Breast Cancer therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
To analyze the effectiveness of these therapies, have calculated their attributed analysis by giving them scores based on their ability to improve atrial and ventricular dimension/function and ability to regulate heart rate.
Further, the therapies’ safety is evaluated wherein the adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials, which directly affects the safety of the molecule in the upcoming trials. It sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the route of administration, order of entry and designation, probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
TNBC Market Access and Reimbursement
Pfizer Oncology Together TALZENNA.
Pfizer Oncology Together offers personalized support to patients and families throughout their TALZENNA treatment. The program helps identify financial assistance options to lower out-of-pocket costs, regardless of insurance status. It also connects patients and caregivers with resources to manage daily challenges during treatment. Eligible commercially insured patients may qualify for Pfizer’s Co-pay Assistance Program, potentially paying as little as USD 0 per month, with up to USD 10,000 in annual savings per product. However, those enrolled in government-funded insurance programs like Medicare, Medicaid, TRICARE, or Veterans Affairs Health Care are not eligible for this co-pay assistance program.
Further details will be provided in the report.
The TNBC Market report provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenarios, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Scope of the TNBC Market Report
- The Triple Negative Breast Cancer market report covers a segment of key events, an executive summary, and a descriptive overview of triple negative breast cancer (TNBC), explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight into the epidemiology segments and forecasts, the future growth potential of incidence rate, disease progression, and treatment guidelines have been provided.
- Additionally, an all-inclusive account of the current and emerging therapies and the elaborative profiles of late-stage and prominent therapies will impact the current treatment landscape.
- A detailed review of the triple negative breast cancer market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The TNBC treatment market report provides an edge while developing business strategies by understanding trends through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help shape and drive the 7MM TNBC.
Triple Negative Breast Cancer Market report insights
- Triple Negative Breast Cancer Patient Population
- Therapeutic Approaches
- TNBC Pipeline Analysis
- TNBC Market Size
- Triple Negative Breast Cancer Market Trends
- Existing and Future Triple Negative Breast Cancer Market Opportunity
TNBC Market report key strengths
- 10 years Forecast
- The 7MM Coverage
- TNBC Epidemiology Segmentation
- Key Cross Competition
- Attribute Analysis
- Triple Negative Breast Cancer Drugs Uptake
- Key TNBC Market Forecast Assumptions
Triple Negative Breast Cancer Market report assessment
- Current Treatment Practices
- Triple Negative Breast Cancer Unmet Needs
- TNBC Pipeline Product Profiles
- TNBC Market Attractiveness
- Qualitative Analysis (SWOT and Attribute Analysis)
Key Questions
Triple Negative Breast Cancer Market Insights
- What was the total market size of triple negative breast cancer (TNBC), the market size of triple negative breast cancer (TNBC) by therapies, and market share (%) distribution in 2020, and what would it look like by 2034? What are the contributing factors for this growth?
- How will DATROWAY affect the treatment paradigm of TNBC?
- How will KEYTRUDA compete with other upcoming products and marketed therapies?
- Which drug is going to be the largest contributor by 2034?
- What are the pricing variations among different geographies for approved and marketed therapies?
- How would future opportunities affect the market dynamics and subsequent analysis of the associated trends?
TNBC Epidemiology Insights
- What are the disease risks, burdens, and unmet needs of triple negative breast cancer (TNBC)? What will be the growth opportunities across the 7MM with respect to the patient population pertaining to triple negative breast cancer?
- What is the historical and forecasted triple negative breast cancer (TNBC) patient pool in the United States, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan?
- Out of the countries mentioned above, which country would have the highest incident cases of triple negative breast cancer population during the forecast period (2025–2034)?
- What factors are contributing to the growth of triple negative breast cancer cases?
Current Treatment Scenario, Marketed Drugs, and Emerging Therapies
- What are the current options for the treatment of triple negative breast cancer (TNBC)? What are the current clinical and treatment guidelines for treating triple negative breast cancer (TNBC)?
- How many Triple Negative Breast Cancer companies are developing therapies for the treatment of TNBC?
- How many emerging TNBC therapies are in the mid-stage and late stage of development for treating triple negative breast cancer (TNBC)?
- What are the recent novel therapies, targets, mechanisms of action, and technologies developed to overcome the limitations of existing therapies?
- What is the cost burden of current treatment on the patient?
- Patient acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the accessibility issues of approved therapy in the US?
- What is the 7MM historical and forecasted Triple Negative Breast Cancer market?
Reasons to Buy
- The Triple Negative Breast Cancer market report will help develop business strategies by understanding the latest trends and changing treatment dynamics driving the TNBC market.
- Insights on patient burden/ TNBC incidence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- Understand the existing TNBC market opportunities in varying geographies and the growth potential over the coming years.
- The distribution of historical and current Triple Negative Breast Cancer patient share is based on real-world prescription data in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identifying upcoming Triple Negative Breast Cancer companies in the market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the conjoint analysis section to provide visibility around leading classes.
- Highlights of Access and Reimbursement policies for triple negative breast cancer, barriers to accessibility of approved therapy, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing market so that the upcoming TNBC companies can strengthen their development and launch strategy.
Stay Updated with us for Recent Articles:-
- Metastatic HER2-Positive Breast Cancer
- Estrogen Receptor Positive Breast Cancer: Market Outlook
- Global HR+/ HER2- Breast Cancer Market Scenario
- How HR+/ HER2- Breast Cancer Emerging Drugs Will Transform the Market?
- HR-positive/ HER2-negative Breast Cancer Market Insights: Upcoming Therapies and Market Analysis
- Breast Cancer: Understand Your Breasts, Recognize the Symptoms
- Key Facts To Know About Triple-Negative Breast Cancer In The Breast Cancer Awareness Month
- 7 Most Common Myths About Breast Cancer Demystified
- Roche’s HER2-Positive Breast Cancer Treatment Franchise
- Latest DelveInsight Blogs
---Market-.png&w=256&q=75)