Head and Neck cancer Market
- The Head and Neck Cancer marke size is anticiapted to grow with a significant CAGR during the study period (2020-2034).
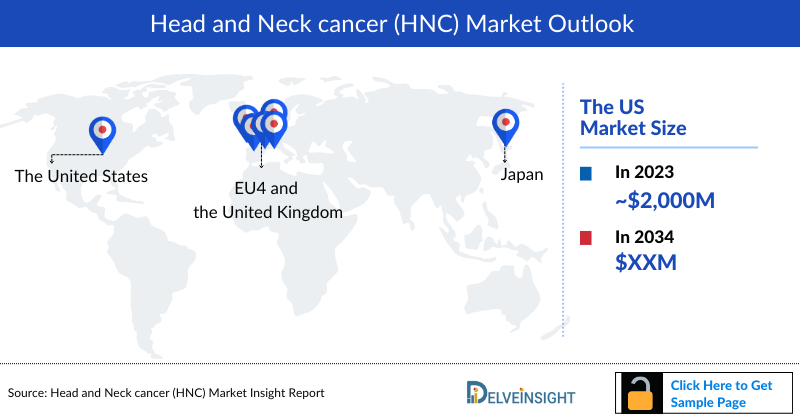
- Among the 7MM, the United States accounted for the largest Head and Neck Cancer market size (more than USD 2,000 million), in comparison to EU4 (Germany, Spain, Italy, France), the United Kingdom, and Japan in 2023.
- Head and Neck Cancer refers to several types of cancers that affect the head and neck areas of the body. These cancers account for approximately 3–5% of all cancers in the US and are common in men and people over the age of 50.
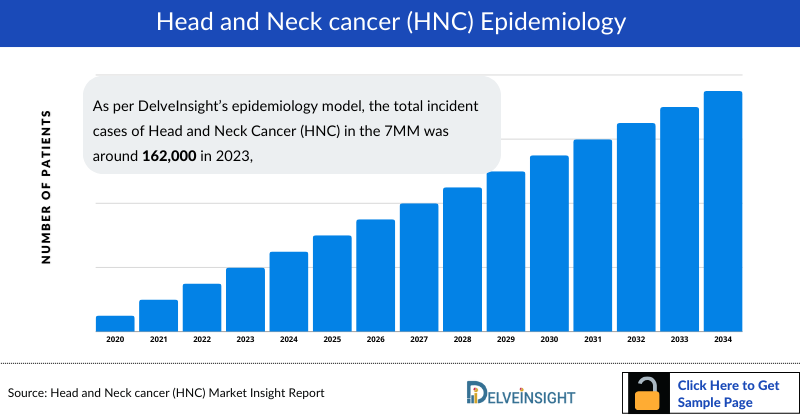
- As per DelveInsight’s epidemiology model, the total incident cases of Head and Neck Cancer (HNC) in the 7MM was around 162,000 in 2023, and the United States showed the highest incident cases of HNC representing ~41% of total cases in the 7MM.
- In 2023, the site-specific incident cases of HNC in the US were maximum for oral and oropharyngeal cancer at nearly 70% cases, followed by laryngeal cancer and hypopharyngeal cancer; nasopharyngeal cancer; salivary gland cancer; and nasal cavity and paranasal sinus cancer.
- Many Head and Neck Cancer can be cured, especially if diagnosed early. Although eliminating the cancer is the primary goal of treatment, preserving the function of the nearby nerves, organs, and tissues is also important.
- Head and Neck Cancer companies are innovating treatment options; notable developers include Merck, Bristol Myers Squibb, AstraZeneca, and PDS Biotech.
- In April 2025, US pharmaceutical company MSD has announced results from its Phase III KEYNOTE-689 trial evaluating Keytruda (pembrolizumab) for the treatment of resected, locally advanced head and neck squamous cell carcinoma (LA-HNSCC). The study showed that Keytruda improved event-free survival (EFS) when used as a perioperative therapy. The randomized, active-controlled trial included 714 participants diagnosed with stage III or IVA resected LA-HNSCC.
- In March 2025, PDS Biotechnology has initiated the Phase III VERSATILE-003 clinical trial, a randomized, multi-center study designed to evaluate the safety and effectiveness of Versamune HPV in combination with pembrolizumab for treating head and neck squamous cell carcinoma (HNSCC). This combination is being developed as a first-line therapy for patients with recurrent or metastatic HPV16-positive HNSCC.
- In December 2023, Exelixis announced the initiation of STELLAR-305, a Phase II/III pivotal trial evaluating zanzalintinib in combination with pembrolizumab versus pembrolizumab alone in patients with previously untreated PD-L1-positive recurrent or metastatic squamous cell carcinoma of the head and neck (SCCHN).
- As per DelveInsight’s estimates, the potential Head and Neck Cancer drugs that can mark a significant change in the market during the forecast period include Leukocyte Interleukin (CEL-SCI), Zanzalintinib + pembrolizumab (Exelixis), and others.
- The current median overall survival for head and neck squamous cell carcinoma (HNSCC) patients in recurrent or metastatic situations is 8 months, underlining the biggest unmet need for additional effective therapies that can increase overall survival for HNSCC patients
DelveInsight's “Head and Neck Cancer Market Insights, Epidemiology and Market Forecast – 2034” report delivers an in-depth understanding of head and neck cancer, historical and forecasted epidemiology as well as the head and neck cancer therapeutics market trends in the United States, EU4 (Germany, Spain, Italy, and France) and the United Kingdom, and Japan.
Head and neck cancer market report provides real-world prescription pattern analysis, emerging drugs, market share of individual therapies, and historical and forecasted 7MM head and neck cancer market size from 2020 to 2034. The Head and Neck Cancer treatment market report also covers current head and neck cancer treatment practices/algorithms and unmet medical needs to curate the best opportunities and assess the Head and Neck Cancer market’s underlying potential.
|
Study Period |
2020 to 2034 |
|
Forecast Period |
2024-2034 |
|
Geographies Covered |
|
|
Head and Neck Cancer Market |
|
|
Head and Neck Cancer Market Size | |
|
Head and Neck Cancer Companies |
CEL-SCI, Junshi Biosciences, Coherus, Exelixis, Immutep S.A.S., Merck Sharp & Dohme LLC, Iovance Biotherapeutics, BeiGene, Akeso, Chia Tai-Tianqing, Kura Oncology, Hoffmann-La Roche, Eli Lilly and Company, and others. |
|
Head and Neck Cancer Epidemiology Segmentation |
|
Head and Neck Cancer Treatment Market
Head and Neck Cancer Overview, Country-Specific Treatment Guidelines and Diagnosis
Head and neck cancer usually begins in the squamous cells – often referred to as squamous cell carcinomas of the head and neck – that line the mucosal surfaces of the head and neck (for example, those inside the mouth, throat, and voice box. They can also begin in the salivary glands, sinuses, muscles, or nerves, but they are less common than squamous cell carcinomas.
Head and neck cancer diagnosis involves a thorough medical history review and physical examination, followed by imaging tests like computed tomography (CT) scan or magnetic resonance imaging (MRI), and confirmation through biopsy or endoscopy for tissue sampling and analysis.
The head and neck cancer treatment market report provides an overview of head and neck cancer pathophysiology, diagnostic approaches, and detailed treatment algorithm along with a real-world scenario of a patient’s journey beginning from the first symptom, the time taken for diagnosis to the entire treatment process.
Further details related to country-based variations in diagnosis are provided in the report...
Head and Neck Cancer Treatment
The Head and Neck Cancer treatment market is expanding due to rising cases, advanced therapies, and increasing global healthcare awareness. HNSCC usually presents at an early or locally advanced stage. Initial therapy with surgery, radiation, or multiple modalities is given with curative intent. Currently, no approved therapy in neo, and adjuvant settings exist, whereas the first-line therapy for recurrent metastatic head and neck squamous cell cancer (R/M-HNSCC) has been revolutionized by the introduction of immune-checkpoint monoclonal antibodies inhibitors (ICIs), a class of drugs targeting the inhibitory immune-checkpoint receptors. KEYNOTE-048 led to the approval of the anti-PD-1 pembrolizumab in a first-line setting, alone or in combination with cisplatin/5 fluorouracil-based chemotherapy. Head and neck cancer clinical trials explore innovative therapies, aiming to improve treatment outcomes and survival rates for affected patients.
Treatment options for R/M HNSCC are limited, especially regarding second-line regimens, mainly referring to chemotherapy, targeted therapy, or a combination of the two. In the CheckMate 141 and KEYNOTE-040 trials, the results showed that ICIs significantly increased OS over standard therapy as second-line treatments. Therefore, NCCN guidelines have recommended both nivolumab and pembrolizumab.
Further details related to country-based variations in treatment are provided in the report...
Head and Neck Cancer Epidemiology
The head and neck cancer epidemiology chapter in the report provides historical as well as forecasted in the 7MM covering the United States, EU4 countries (Germany, France, Italy, and Spain), the United Kingdom, and Japan from 2024 to 2034. The Head and Neck Cancer epidemiology is categorized into comprehensive segments, including Total Incident Cases of Head and Neck Cancer (HNC), HPV-specific Incident Cases, Site-specific Incident Cases, Molecular Alteration-specific Incident Cases, and Stage-specific Incident Cases of Head and Neck Cancer (HNC), offering detailed analytical insights.
- HPV infection is an increasingly common risk factor for head and neck squamous cell carcinoma (HNSCC). HPV infection is associated with most oropharyngeal cancers (>70%) and a small minority of cancers at other anatomical sites in the head and neck. In 2023, approximately 35% of HPV-specific incident cases of head and neck cancer were found to be HPV positive in the United States.
- DelveInsight’s consultant estimates that in 2023, the molecular alteration-specific incident cases of HNC in the US were maximum for TP53 cases followed by CCND1, PIK3CA, NOTCH1, KMT2D, EGFR, NSD1, FGFR1, and HRAS mutations.
- The analysis from 2023 suggests that the stage-specific incident cases of HNC in the US were maximum for regional, with approximately 45% cases, followed by localized cases, distant cases, and cases of the unknown stage.
- Among EU4 and the UK, the highest number of incident cases of head and neck cancer was observed in Germany in 2023, which is followed by France.
Stay ahead with insights on Head and Neck Cancer prevalence and patient population projections.
Head and Neck Cancer Drug Chapters
The drug chapter segment of the head and neck cancer treatment market report encloses a detailed analysis of marketed head and neck cancer drugs and late-stage (Phase III and Phase II) pipeline drugs. It also deep dives into the head and neck cancer clinical trial details, recent and expected market approvals, patent details, the latest news, and recent deals and collaborations. The Head and Neck Cancer drugs market is expanding rapidly due to rising cases, advanced therapies, and increased research investments.
Marketed Head and Neck Cancer Drugs
OPDIVO (nivolumab): Bristol Myers Squibb
OPDIVO (nivolumab) is a programmed death-1 (PD-1) immune checkpoint inhibitor designed to uniquely harness the body’s immune system to help restore anti-tumor immune response. In November 2016, the US FDA approved nivolumab (OPDIVO Injection) for the treatment of patients with recurrent or metastatic squamous cell carcinoma of head and neck (SCCHN) with disease progression on or after platinum-based therapy.
KEYTRUDA (pembrolizumab): Merck Sharp & Dohme LLC
KEYTRUDA (pembrolizumab) is an anti-programmed death receptor-1 (PD-1) therapy that works by increasing the ability of the body’s immune system to help detect and fight tumor cells. In June 2019, the FDA approved pembrolizumab (KEYTRUDA) for the first-line treatment of patients with metastatic or unresectable recurrent head and neck squamous cell carcinoma (HNSCC).
|
Drugs |
Company |
MOA |
ROA |
Approval |
|
KEYTRUDA (Pembrolizumab) |
Merck |
Programmed death receptor-1 (PD-1)-blocking antibody |
Intravenous |
US: June 2019 EU: November 2019 JP: December 2019 |
|
OPDIVO (nivolumab) |
Bristol-Myers Squibb |
Programmed death receptor-1 (PD-1)-blocking antibody |
Intravenous |
US: November 2016 EU: March 2017 JP: April 2017 |
Stay ahead with key updates on Head and Neck Cancer treatments. Access the 2025 pipeline report for exclusive insights!
Emerging Head and Neck Cancer Drugs
Leukocyte Interleukin (MULTIKINE): CEL-SCI
MULTIKINE is an immunotherapeutic agent that is being developed as a potential first-line neoadjuvant therapy in patients with advanced primary squamous cell carcinoma of the head and neck (SCCHN). In 2007, CEL-SCI’s MULTIKINE has received ODD as neoadjuvant therapy in patients with SCCHN by the FDA. Currently, it is being evaluated under global Phase III clinical trial.
Zanzalintinib + pembrolizumab: Exelixis
Zanzalintinib is a next-generation oral tyrosine kinase inhibitor that inhibits the activity of receptor tyrosine kinases implicated in cancer growth and spread, including VEGF receptors, MET, AXL and MER. Currently, it is being evaluated in a Phase II/III trial of zanzalintinib in combination with pembrolizumab versus zanzalintinib-matched placebo in combination with pembrolizumab in subjects with PD-L1 positive recurrent or metastatic head and neck squamous cell carcinoma (R/M HNSCC) incurable by local therapies who have not received prior systemic therapy for recurrent or metastatic disease.
Head and Neck Cancer Market Outlook
Key Head and Neck Cancer companies, such as Exelixis, CEL-SCI, and others are evaluating their lead candidates in different stages of clinical development, respectively. They aim to investigate their products for the treatment of head and neck cancer.
As per DelveInsight’s patient-based forecasting model, the United States accounted for nearly 65% of the total Head and Neck Cancer market size among the 7MM in 2023.
KEYTRUDA (pembrolizumab) monotherapy achieved the largest Head and Neck Cancer market share among all the available Head and Neck Cancer therapies in 2023 in the 7MM.
The Head and Neck Cancer market size is expected to grow due to rising incidence rates globally, driven by factors like tobacco use and HPV infection, along with advancements in treatment options including targeted therapies and immunotherapies.
Head and Neck Cancer Drugs Uptake
This section focuses on the uptake rate of potential Head and Neck Cancer drugs expected to be launched in the Head and Neck Cancer market during 2024–2034, which depends on the competitive landscape, safety, and efficacy data along with order of entry. It is important to understand that the key Head and Neck Cancer companies evaluating their novel therapies in the pivotal and confirmatory trials should remain vigilant when selecting appropriate comparators to stand the greatest chance of a positive opinion from regulatory bodies, leading to approval, smooth launch, and rapid uptake.
Further detailed analysis of emerging therapies drug uptake in the report...
Head and Neck Cancer Pipeline Activities
The Head and Neck Cancer therapeutics market report provides insights into different Head and Neck Cancer clinical trials within Phase III and Phase II stages. It also analyzes key Head and Neck Cancer companies involved in developing targeted therapeutics.
Head and Neck Cancer Pipeline Drugs Market
The Head and Neck Cancer drugs market report covers information on collaborations, acquisitions and mergers, licensing, and patent details for emerging head and neck cancer therapies.
KOL Views on Head and Neck Cancer
To keep up with the real-world scenario in current and emerging Head and Neck Cancer market trends, we take opinions from Key Industry leaders working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts were contacted for insights on the evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake along with challenges related to accessibility.
DelveInsight’s analysts connected with 20+ KOLs to gather insights; however, interviews were conducted with 10+ KOLs in the 7MM. Centers such as Winship Cancer Institute, Institute of Cancer Research, National Cancer Center Hospital, etc., were contacted. Their opinion helps understand and validate current and emerging treatment patterns of head and neck cancer. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
|
Region |
KOL Views |
|
United States |
“It is seen in the clinical trials that the combination of immunotherapy along with SOC (chemotherapy/radiotherapy) have not shown much promising results in HNC. There should be more focus research in the areas of treatment and understand where the treatment has too started in the neo adjuvant, adjuvant settings or during the radiation.” |
|
Japan |
“Now a days trials are combining other agents with already existing PD-1 inhibitors to improve the response rates because on 1 out of 5 patients respond to PD-1 inhibitors. Researchers are trying to search new and innovative therapies, combination therapies are also being investigated in the hope to prolong the OS and PFS.” |
Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT analysis and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of gaps in disease diagnosis, patient awareness, physician acceptability, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided.
Conjoint Analysis analyzes multiple approved and emerging Head and Neck Cancer therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
In efficacy, the trial’s primary and secondary outcome measures are evaluated. A few of the important primary endpoints were overall survival (OS), progression-free survival (PFS), overall response rate (ORR), etc.
Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Head and Neck Cancer Market Access and Reimbursement
OPDIVO (nivolumab)
OPDIVO is a PD-1-blocking antibody approved for the treatment of patients with recurrent or metastatic squamous cell carcinoma of the head and neck who have progressed on or after platinum-based therapy. Medicare is applicable for OPDIVO. Medicare supplemental insurance, also known as Medigap coverage, is an optional plan that can be used to help cover a portion of Medicare costs such as copayments or out-of-pocket expenses. Medicare beneficiaries can expect to pay between USD 0 and USD 8,210 per infusion. If a patient has a Medigap plan, he or she can reduce his or her share of the medication cost even further.
KEYTRUDA (pembrolizumab)
Patients with Medicare may or may not have to pay a portion of the cost of KEYTRUDA (pembrolizumab) based on their insurance plan. For example, with a Medicare Advantage plan, 41% of patients had no out-of-pocket costs for the 200 mg dose of KEYTRUDA. Roughly 80% of patients responsible for a portion of the cost paid between USD 0 and USD 925 per infusion after meeting their deductible. Most patients with Medicaid typically pay USD 4–USD 8 per KEYTRUDA infusion. The co-pay part which needs to be paid by the patient ranges from USD 12,045.
The Head and Neck Cancer market segment of key events, an executive summary, descriptive overview of head and neck cancer, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight has been provided into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, and disease progression along with country specific treatment guidelines.
- Additionally, an all-inclusive account of both the current and emerging therapies, along with the elaborative profiles of late-stage and prominent therapies, will have an impact on the current treatment landscape.
- A detailed review of the head and neck cancer market, historical and forecasted Head and Neck Cancer market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The Head and Neck Cancer treatment market report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help in shaping and driving the 7MM head and neck cancer market.
Head and Neck Cancer Market Report Insights
- Head and Neck Cancer Patient Population
- Head and Neck Cancer Therapeutic Approaches
- Head and Neck Cancer Pipeline Analysis
- Head and Neck Cancer Market Size
- Head and Neck Cancer Market Trends
- Existing and Future Head and Neck Cancer Market Opportunity
Head and Neck Cancer Market Report Key Strengths
- Eleven-year Forecast
- 7MM Coverage
- Head and Neck Cancer Epidemiology Segmentation
- Inclusion of Country Specific Treatment Guidelines
- KOL’s Feedback On Approved and Emerging Therapies
- Key Cross Competition
- Conjoint Analysis
- Head and Neck Cancer Drugs Uptake
- Key Head and Neck Cancer Market Forecast Assumptions
Head and Neck Cancer Market Report Assessment
- Current Head and Neck Cancer Treatment Practices
- Head and Neck Cancer Unmet Needs
- Head and Neck Cancer Pipeline Product Profiles
- Head and Neck Cancer Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
- Head and Neck Cancer Market Drivers
- Head and Neck Cancer Market Barriers
FAQs
- What is the growth rate of the 7MM head and neck cancer treatment market?
- What was the head and neck cancer market size, the market size by therapies, market share (%) distribution in 2020, and what would it look like in 2034? What are the contributing factors/key catalysts for this growth?
- Is there any unexplored patient setting that can open the window for growth in the future?
- What are the pricing variations among different geographies for approved and off-label Head and Neck Cancer therapies?
- How would the market drivers, barriers, and future opportunities affect the Head and Neck Cancer market dynamics and subsequent analysis of the associated trends?
- What are the current and emerging options for the treatment of head and neck cancer?
- How many Head and Neck Cancer companies are developing therapies for the treatment of head and neck cancer?
- What are the recent novel therapies, targets, Head and Neck Cancer mechanisms of action, and technologies developed to overcome the limitations of existing Head and Neck Cancer therapies?
- Patient/physician acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the country-specific accessibility issues of expensive, recently approved Head and Neck Cancer therapies?
Reasons to buy Head and Neck Cancer Market Forecast Report:
- The Head and Neck Cancer drugs market report will help in developing business strategies by understanding the latest trends and changing treatment dynamics driving the head and neck cancer market.
- Insights on patient burden/Head and Neck Cancer prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years
- Understand the existing market opportunities in varying geographies and the growth potential over the coming years.
- Distribution of historical and current Head and Neck Cancer patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identifying strong upcoming Head and Neck Cancer companies in the market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging Head and Neck Cancer therapies under the conjoint analysis section to provide visibility around leading classes.
- Highlights of access and reimbursement policies of approved Head and Neck Cancer therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing market so that the upcoming Head and Neck Cancer companies can strengthen their development and launch strategy.
-market.png&w=256&q=75)



-market.png&w=256&q=75)


