
Riociguat potential benefit
- Home
- ATS Conference
- pde5i in pah
PDE5i in PAH: ATS 2022
Pulmonary hypertension encompasses a heterogeneous group of disorders with the common feature of elevated pulmonary vascular resistance. Pulmonary hypertension is a progressive disease with treatment focused on management of symptoms and treatment of underlying diseases. The market size of Chronic Pulmonary Hypertension in the 7MM countries (the US, EU-5 (Germany, France, Italy, Spain, the UK), and Japan) was USD 26 billion in 2019. According to DelveInsight’ analysts the market is expected to reach > USD 500 billion in 2032.
Calcium channel blockers (CCBs), endoserin receptor antagonists (ERAs), and phosphodiesterase-5 (PDE-5) inhibitors are used in the current market as pharmacological treatments for pulmonary arterial hypertension (PAH). Phosphodiesterase-5 (PDE-5) inhibitors, such as sildenafil and tadalafil, have antiproliferative effects on vascular smooth muscle cells and are effective in inducing vascular dilation. Endothelin receptor antagonists such as ambrisentan, bosentan, and macitentan exert vasoconstrictor and thread division-inducing effects on pulmonary vascular smooth muscle. Phosphodiesterase type-5 (PDE-5) inhibitors and guanylate cyclase (sGC) stimulants have been shown to reduce the levels of enzymes involved in the production of NO, a natural substance in the body that relaxes pulmonary blood vessels. NO increases the intracellular level of cyclic guanosine monophosphate GMP (cyclic GMP). Therefore, another established therapeutic approach is the use of drugs known as PDE-5 inhibitors to inhibit cyclic GMP degradation.
Bayer`s Riociguat is an oral drug referred to as a soluble guanylate cyclase stimulant accredited for the remedy of patients with pulmonary arterial hypertension (PAH) and persistent/recurrent chronic thromboembolic pulmonary hypertension (CTEPH). The efficacy and protection of riociguat in sufferers with PAH has been established in an essential PATENT1 study, leading to the approval of riociguat for the treatment of PAH in lots of countries. Patients treated with Adempas (Riociguat) had a significant delay in time and fewer occasions of medical deterioration in comparison to sufferers handled with placebo (p = 0.0046; stratified log-rank test).
Riociguat and the phosphodiesterase-5 inhibitor (PDE-5i), approved for the treatment of pulmonary arterial hypertension (PAH), act by the same pathway through different mechanisms. Riociguat may be an alternative option for PAH patients who do not respond appropriately to treatment with PDE-5i, but a comparison of the potential benefits of riociguat and PDE5i in these patients is needed. REPLACE study results shown clinical improvement in 24 weeks. This was defined as a pre-determined improvement in 2 of the 3 parameters (6MGT, WHO-FC, and / or NTproBNP) and absence of clinical worsening that is 1% in Adempas and 9% in patients who continued PDE5i treatment. WHO FC on Adempas vs. those continuing treatment with the PDE5i change shows 2% worsened in Adempas and 4% worsened in PDE-5i.
Five year total revenue of Riociguat
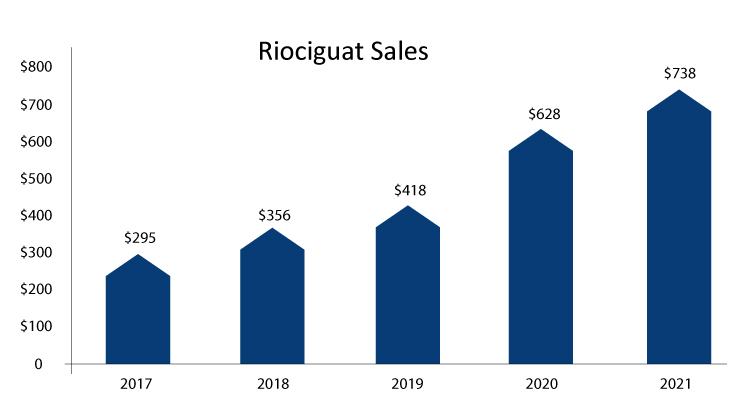
The only class approved for the treatment of CTEPH is Riociguat. A PATENT1 study found that riociguat had a significant effect on patients with PAH. The CHEST1 study evaluated riociguat in patients with CTEPH and found that it improved motor performance, PVR and WHO functional classes in patients with unresectable or persistent / recurrent CTEPH, with monotherapy or endothelin receptor antagonists. REPLACE study have shown that results in Adempas, as monotherapy or in combination with endothelin receptor antagonists improves exercise capacity for the treatment of adult patients with pulmonary arterial hypertension (PAH) WHO functional class (FC) II-III. Efficacy has also been demonstrated in PAH populations that include the etiology of PAH associated with idiopathic or hereditary PAH or connective tissue disorders.
Adempas is expected to grow exponentially in terms of sales. The company is in recruiting phase for multiple trials such as JPMS-PAH study, JPMS-CTEPH study, THERAPY-HYBRID-BPA Trial, ROAR, and the aim of these study is to collect post-marketing information on Riociguat safety to maintain its growth all over.
Executive Summary
Bayer`s Riociguat is in clinical developement for patients with pulmonary arterial hypertension (PAH) and persistent/recurrent chronic thromboembolic pulmonary hypertension (CTEPH).