Download Case Study to Unlock Valuable Insights
Fill out the form to gain access to exclusive content and data-driven strategies

Competitive Intelligence News Monitoring: Pharma CI, Regulatory Insights, and 7MM Market Surveillance
- Home
- case study
- ci news monitoring case study
Objective
In today’s fast-paced pharmaceutical and life sciences industry, staying competitive requires more than innovative R&D, it demands real-time Competitive Intelligence (CI) and proactive market and regulatory insights. With rapid shifts in drug development pipelines, competitor strategies, and regulatory landscapes across global markets such as the 7MM (Seven Major Markets), companies must remain vigilant to make informed decisions.
DelveInsight’s pharma CI news monitoring services equip clients with timely updates on market trends, clinical trial advancements, regulatory approvals, and competitor actions, allowing them to identify new opportunities, mitigate risks, and optimize strategic planning. Clients have used these insights to refine product development, anticipate competitive threats, and strengthen their commercial strategy.
Problem Statement
A mid-sized US-based pharmaceutical company required real-time and comprehensive intelligence on all commercial, clinical, and regulatory developments related to Drug X in the 7MM region. Their key needs included:
- Daily tracking of drug development news, competitor activities, and regulatory updates for Drug X.
- Immediate same-day alerts for any high-impact news posing competitive or commercial threats.
- A consolidated monthly Competitive Intelligence and news monitoring report covering all relevant developments.
- Insights on business implications, enabling proactive decision-making.
The client wanted complete visibility into every action taken by competitors or stakeholders around Drug X across the US, EU5, and Japan, with zero delays in high-priority alerts.
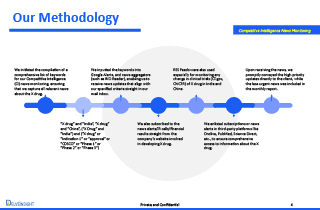
Our Methodology
To deliver accurate, comprehensive, and real-time insights, DelveInsight implemented a multi-layered Competitive Intelligence news monitoring framework:
1. Strategic Keyword Mapping for CI Monitoring
- “Drug X” + “7MM”
- “Drug X” + (Indication / clinical phase / approval / regulatory body)
- “Drug X” + (“Phase 1”, “Phase 2”, “Phase 3”, “FDA approval”, “EMA”, “CDSCO”)
- Competitor names, pipeline terms, and relevant therapeutic areas
This ensured full coverage of all clinical, regulatory, scientific, and commercial updates.
2. Real-Time Alerts via Trusted Sources
We configured Google Alerts, news aggregators like Inoreader, and other global monitoring tools to source timely updates directly into our CI workflows.
3. Direct Intelligence from Company Sources
To capture insider and early insights, we subscribed to:
- Company press releases
- Investor relations (IR) updates
- Earnings and financial results
- Corporate presentations and announcements
4. Clinical Trial Surveillance Across Global Registries
We tracked real-time changes in clinical trial activity using:
- ClinicalTrials.gov (CT.gov)
- ChiCTR
- EU Clinical Trials Register
- Additional regional databases as needed
5. Scientific & Medical Literature Monitoring
We incorporated updates from platforms such as:
- PubMed
- ScienceDirect
- OncLive
- Other specialty medical databases
6. Priority-Based Client Communication
- High-priority alerts were sent immediately (same-day).
- Low-urgency updates were compiled into an in-depth monthly CI report, complete with analysis and business impact assessment.
Outcome
DelveInsight’s structured, technology-driven CI methodology delivered significant value for the client by providing:
- Comprehensive Coverage of Drug X Across 7MM
With an advanced keyword ecosystem and multi-platform tracking, we ensured 100% visibility into all relevant developments—clinical, regulatory, commercial, or competitive.
- Real-Time Competitive Alerts
Google Alerts, Inoreader, RSS feeds, and direct company subscriptions enabled rapid alerts, helping the client stay ahead of emerging threats and opportunities.
- Deeper Insights into Market and Competitor Dynamics
By analyzing news, IR updates, and clinical progress, we offered insights into:
-
- Competitor strategy shifts
- Regulatory risks
- Pipeline progression
- Market entry windows
Enhanced Strategic Decision-Making
With fast and reliable intelligence, the client:
- Adjusted product development strategies
- Identified early opportunities in 7MM markets
- Strengthened commercial planning
- Mitigated risks associated with competitor moves
Strengthened Client Partnership
Our commitment to timely updates and actionable intelligence positioned DelveInsight as a trusted CI partner delivering accurate, high-value, and ready-to-use insights for strategic decision-making.
Our Related Services
Sample Visuals

Get Visuals