Download Case Study to Unlock Valuable Insights
Fill out the form to gain access to exclusive content and data-driven strategies

COVID-19 Pipeline Intelligence: Competitive Assessment of Developmental Candidates
- Home
- case study
- competitive landscape of covid 19 therapies
Objective: Identifying Market Opportunities in the Evolving COVID-19 Therapeutic Space
The strategic assessment conducted by DelveInsight Business Research focused on evaluating the developmental pipeline of COVID-19 therapeutics to provide comprehensive competitive intelligence and identify emerging opportunities. The objective was to map the landscape of clinical-stage candidates, understand their differentiation potential, and enable informed strategic decisions regarding competitive positioning, partnership opportunities, and market entry timing.
Problem Statement: Navigating a Complex and Evolving Pipeline
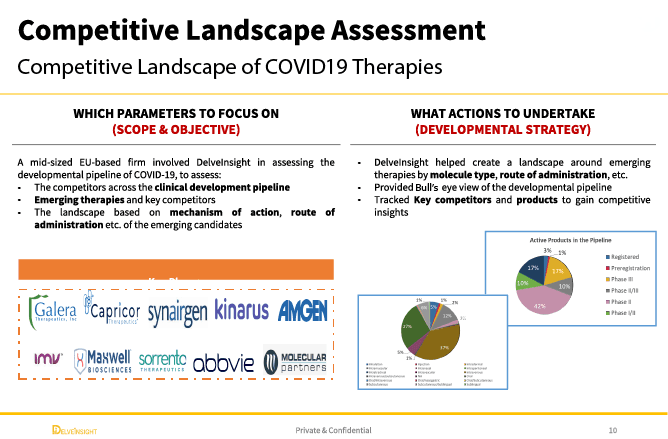
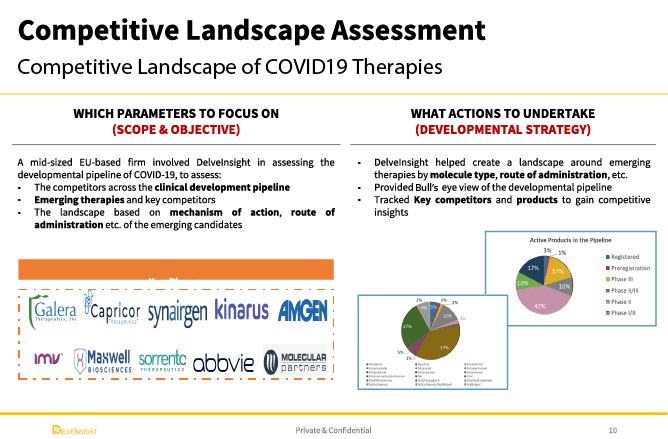
A leading mid-sized EU-based pharmaceutical firm faced a critical strategic challenge: How to identify and evaluate emerging COVID-19 therapies within an increasingly crowded developmental pipeline. The company sought to understand the competitive landscape across multiple dimensions, including mechanism of action, route of administration, and clinical development stage, to assess where emerging candidates could create competitive advantage, identify partnership opportunities, or highlight potential threats to existing market positioning.
The key challenge was moving beyond surface-level pipeline tracking to gain actionable intelligence: Which emerging therapies demonstrate true clinical differentiation? Who are the key competitors developing next-generation solutions? How do pipeline candidates position against established standards-of-care? The decision required rigorous, multi-dimensional pipeline analysis to inform strategic resource allocation and competitive response planning.
Our Methodology: Comprehensive Pipeline and Competitive Intelligence
DelveInsight applied a sophisticated, multi-layered methodology to deliver actionable competitive intelligence:
1. Therapeutic R&D Developmental Pipeline Assessment
Conducted extensive mapping of COVID-19 candidates across all clinical development stages (Phase I through Phase III/IV). Analyzed historical development timelines, regulatory pathways, and clinical trial progression to assess likelihood of regulatory approval and market entry.
2. Assessment of the Drugs
Evaluated emerging therapeutic candidates on key differentiation parameters including efficacy data, safety profiles, clinical endpoints, and pharmacological characteristics. Compared candidate profiles against established COVID-19 treatments to identify innovation potential and clinical advantages.
3. Key Pharmaceuticals Portfolio Assessment
Analyzed the strategic portfolios of leading players and emerging biotech firms active in COVID-19 development. Assessed each competitor's pipeline depth, therapeutic positioning, and strategic focus areas to understand competitive dynamics and potential partnership or acquisition targets.
4. Strategic Planning Assistance
Provided framework for strategic decision-making by integrating pipeline intelligence with competitive positioning analysis, enabling clients to identify where competitive advantage exists, assess market timing, and evaluate partnership or acquisition opportunities.
Results: Actionable Competitive Intelligence and Strategic Roadmap
The comprehensive assessment delivered a suite of outputs that transformed pipeline data into strategic insight:
Detailed Profiling of Active Products in the Pipeline
Created comprehensive candidate profiles encompassing clinical development stage, mechanism of action, route of administration, clinical endpoints, safety data, regulatory status, and development timelines. Each profile positioned the candidate within the competitive landscape and assessed its differentiation potential relative to existing and emerging alternatives.
Landscape Analysis of Mechanisms of Action and Routes of Administration
Conducted systematic analysis of the therapeutic approaches being pursued across the pipeline, identifying dominant mechanisms (e.g., antivirals, immunomodulators, monoclonal antibodies) and delivery platforms (oral, intravenous, inhaled). Mapped how emerging candidates cluster by therapeutic approach and identified white space opportunities where limited development activity suggested unmet needs or underexplored mechanisms.
Matrix Analysis: Top 10 Competitors Positioned and Ranked
Developed strategic positioning matrix placing the top 10 pharmaceutical companies and biotech firms researched across key competitive dimensions. This visualization enabled rapid assessment of which competitors represented direct threats, potential partners, or acquisition opportunities. The matrix highlighted competitive concentration, strategic differentiation, and areas of emerging competition.
Competitive Appraisal Enabling Strategic Response Planning
Delivered comprehensive overview of all top-tier companies active in COVID-19 therapeutic development, enabling the client to:
- Identify Direct Competitors: Understand which organizations pose the greatest competitive threat based on pipeline depth, regulatory progress, and market positioning.
- Assess Partnership Potential: Evaluate potential collaboration opportunities with companies whose therapeutic approaches complement core capabilities or fill gaps in the client's portfolio.
- Monitor Competitive Threats: Track emerging competitors whose candidates demonstrate strong clinical potential and could disrupt market dynamics.
- Benchmark Strategic Positioning: Compare the client's own pipeline positioning against key competitors to inform resource allocation and strategic prioritization decisions.
Strategic Outcome: From Pipeline Data to Competitive Clarity
The assessment enabled the mid-sized EU-based firm to transition from pipeline observation to strategic action. By synthesizing developmental candidate profiles, competitive positioning data, and market landscape intelligence, DelveInsight provided:
- Clear visibility into the competitive pipeline architecture and which emerging therapies represented genuine clinical innovation versus incremental improvements.
- Actionable competitive intelligence on key players, enabling informed decisions about partnership development, competitive monitoring, and resource allocation priorities.
- Strategic roadmap for evaluating emerging opportunities and threats in the COVID-19 therapeutic space, supporting faster decision-making in a rapidly evolving market.
Conclusion: Pipeline Intelligence as Strategic Advantage
For organizations operating in fast-moving therapeutic areas like COVID-19, comprehensive pipeline assessment delivers critical strategic value. By combining detailed drug profiling, competitive benchmarking, and market landscape analysis, DelveInsight enabled the client to move from data overwhelm to strategic clarity.
In the dynamic COVID-19 marketplace, where therapeutic innovation, competitive threats, and market access dynamics evolve rapidly, having systematic competitive intelligence and pipeline visibility proves essential. DelveInsight's comprehensive assessment provided the foundation for informed strategic decisions about competitive positioning, partnership opportunities, and resource allocation enabling the client to navigate the complex landscape of emerging COVID-19 therapeutics with confidence and strategic precision.
Our Related Services
Sample Visuals
Get Visuals