Acne Vulgaris Market
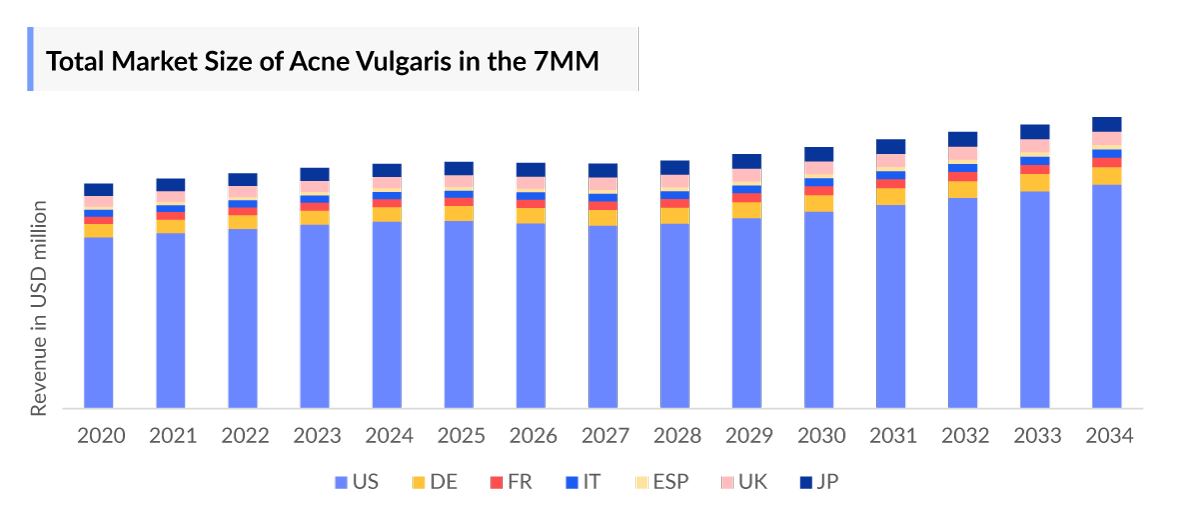
- In 2023, the Acne Vulgaris Market Size was highest in the US among the 7MM, accounting for approximately USD 2,949 million which is further expected to increase by 2034.
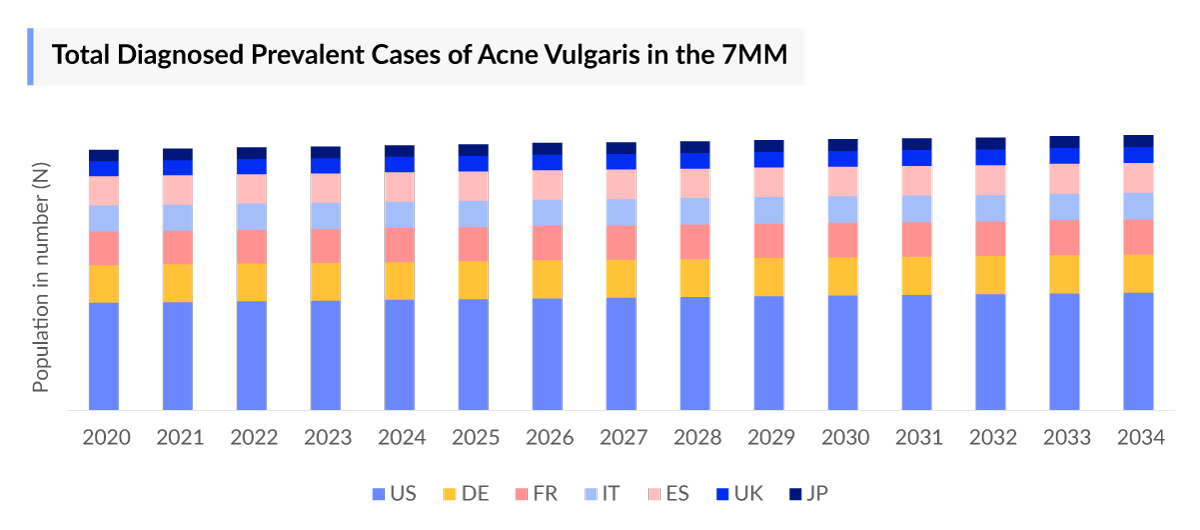
- In 2023, there were approximately 29,433 thousand Acne Vulgaris Diagnosed Prevalent Cases in the 7MM, these cases are further expected to increase during the forecast period (2024–2034). Factors contributing to the rise of diagnosed prevalent cases of acne vulgaris include hormonal changes, dietary influences, genetic predisposition, and environmental factors such as pollution.
- The Acne Vulgaris Age-specific prevalent cases are divided into the following groups: 15-19 years, 20-29 years, 30-39 years, 40-49 years, and 50 years and older. In the United States, a higher number of cases were observed in the age group 15-19 years compared to others, comprising around 5,853 thousand cases in 2023.
- The emerging drug SB204 is expected to launch in the US market by 2027, the drug has the potential to reduce the disease burden of acne vulgaris in the forecasted years.
Request for Unlocking the CAGR of the “Acne Vulgaris Drugs Market”
DelveInsight's “Acne Vulgaris Market Insights, Epidemiology, and Market Forecast – 2034” report delivers an in-depth understanding of the Acne Vulgaris, historical and forecasted epidemiology as well as the Acne Vulgaris therapeutics market trends in the United States, EU4 (Germany, Spain, Italy, and France) and the United Kingdom, and Japan.
Acne Vulgaris Drugs Market Report provides current treatment practices, emerging drugs, and market share of the individual therapies, current and forecasted 7MM Acne Vulgaris market size from 2020 to 2034. The report also covers current Acne Vulgaris Treatment Market practices/algorithms and unmet medical needs to curate the best of the opportunities and assess the underlying potential of the market.
|
Study Period |
2020–2034 |
|
Forecast Period |
2024–2034 |
|
Geographies Covered |
|
|
Acne vulgaris Epidemiology |
|
|
Acne vulgaris Drugs Market |
|
|
Acne Vulgaris Market Analysis |
|
|
Acne vulgaris Companies |
|
|
Future opportunity |
The development of breakthrough therapies, disease-modifying treatments, increased awareness and access to care, and personalized medicine may open up new avenues for targeted treatments, providing further opportunities for market growth. |
Key Factors Driving the Acne Vulgaris Market
Rising Acne Vulgaris Prevalence
The rising prevalence of acne vulgaris due to changes in lifestyle, diet, increased psychological stress, and hormonal changes, especially in the adolescent population, creates a larger market demand for the treatment of acne vulgaris.
Technological Breakthroughs Enhancing Acne Treatment
Technological advancements in skincare and dermatological therapies have enhanced patient outcomes and treatment efficacy, allowing for more precise patient care and more efficient therapies, particularly given the chronic nature of acne.
Emergence of Novel Acne Vulgaris Drugs
Some of the acne vulgaris drugs in clinical trials include SB204 (Pelthos Therapeutics), DMT310 (Dermata Therapeutics), BTX 1503 (Botanix Pharmaceuticals), and B244 (AOBiome), among others.
Acne Vulgaris Treatment Market
Acne vulgaris is a chronic inflammatory dermatological condition characterized by pimples, blackheads, and whiteheads on the skin, primarily affecting pilosebaceous units across various age groups, with adolescents being the most commonly affected demographic. The severity of acne can be classified into mild, moderate, and severe based on the presence of non-inflammatory and inflammatory lesions, erythema, and scarring. The pathogenesis involves a multifactorial interplay of hormonal influences, sebum production, follicular hyperkeratinization, bacterial proliferation, and inflammation, exacerbated by genetic predisposition, medications, and environmental factors. Key symptoms include comedones, inflammatory lesions, and scarring. The management of acne is challenging due to various risk factors, emphasizing the need for comprehensive understanding and targeted interventions. The acne treatment market growth is driven by increasing demand for advanced skincare solutions and emerging non-invasive treatment options worldwide.
Acne Vulgaris Diagnosis
Acne Vulgaris Diagnosis typically involves a thorough clinical examination of the skin to assess the type, severity, and distribution of lesions. In some instances, additional diagnostic procedures are employed to rule out other skin conditions or determine bacterial involvement. These procedures include skin biopsies and microbial cultures. Furthermore, additional tests such as microbial testing, hormonal evaluations, and liver function tests may be conducted in specific cases to provide a comprehensive understanding of the condition.
Further details related to diagnosis are provided in the report…
Acne Vulgaris Treatment
The topical Acne treatments market is expanding with innovative therapies targeting acne's root causes, offering effective solutions for skincare. Current acne treatments face significant limitations, including side effects and the need for long-term use. Retinoids, for example, can cause skin irritation, dryness, and photosensitivity, while antibiotics may lead to resistance, and hormonal therapies are not suitable for all patients. Chemical peels, laser treatments, and isotretinoin, while effective, can be harsh on the skin and require careful monitoring. There is a critical need for innovative treatments that are both effective and safe for prolonged use, with minimal adverse effects.
Non-invasive treatments with minimal side effects, such as light-based therapies or topical probiotics, are needed to provide safer alternatives that can be used by a broader population without the risk of severe adverse effects.
Furthermore, acne vulgaris varies significantly among individuals in terms of severity, skin type, and underlying causes, making a one-size-fits-all approach inadequate. Personalized medicine, which tailors treatment based on genetic, environmental, and lifestyle factors, can significantly improve outcomes. Advances in genomics and artificial intelligence hold the potential to revolutionize acne management by developing customized treatment plans that optimize efficacy and minimize side effects. Addressing these unmet needs is essential for enhancing the standard of care and achieving sustainable, long-term management of acne.
Acne Vulgaris Epidemiology
As the market is derived using a patient-based model, the Acne Vulgaris epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by, Prevalent Cases of Acne Vulgaris, Diagnosed Prevalent Cases of Acne Vulgaris, Severity-specific Diagnosed Prevalent Cases of Acne Vulgaris, Gender-specific Diagnosed Prevalent Cases of Acne Vulgaris, and Age-specific Diagnosed Prevalent Cases of Acne Vulgaris in the 7MM covering, the United States, EU4 countries (Germany, France, Italy, and Spain), United Kingdom, and Japan from 2020 to 2034.
- In 2023, an estimated ~140,217 thousand Acne Vulgaris Prevalence Cases occurred across the 7MM, with approximately 69,500 thousand cases attributed to the US alone. These numbers are expected to increase steadily throughout the forecast period.
- The categorization based on gender, showed that Acne Vulgaris diagnosed prevalent cases in females were higher than in males in the 7MM. The female-diagnosed prevalent cases accounted for 63% of the total cases in the 7MM.
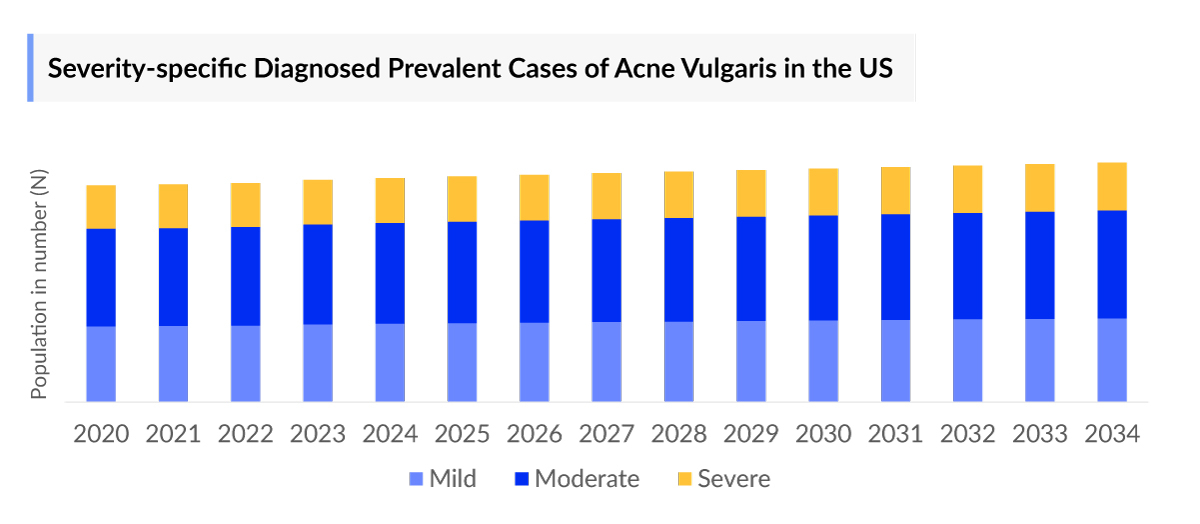
- The Acne Vulgaris Prevalence Cases were sub-segmented further based on the severity of the acne as mild, moderate, and severe. In 2023, the highest diagnosed prevalent cases of acne vulgaris were reported as moderate (45%), whereas there were least cases in the severe (10%) segment in the 7MM.
Acne Vulgaris Drugs Market Chapters
The drug chapter segment of the Acne Vulgaris Drugs Market report encloses a detailed analysis of Acne Vulgaris off-label drugs and late-stage (Phase-III and Phase-II) Acne Vulgaris pipeline drugs. It also helps to understand the Acne Vulgaris clinical trials details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug, and the latest Acne Vulgaris news and press releases.
Acne Vulgaris Marketed Drugs
- AKLIEF (Trifarotene)/ CD-5789: Galderma S.A.
Trifarotene, a first-in-class fourth-generation topical retinoid, has been approved by the FDA for the treatment of acne vulgaris in patients aged 9 years and older. This novel retinoid selectively targets retinoic acid receptor gamma (RAR?), the most common RAR found in the skin, making it a more targeted and skin-specific treatment compared to earlier-generation retinoids. Trifarotene is effective in reducing inflammatory lesions on the face and trunk, with significant improvements observed as early as 2 weeks on the face and 4 weeks on the back, shoulders, and chest. The cream is well tolerated, with common adverse reactions including application-site irritation, pruritus, and sunburn. In June 2017, Mayne Pharma and Galderma entered a global licensing agreement for trifarotene, with Galderma retaining rights to develop and commercialize the drug for common dermatological indications like acne vulgaris while Mayne Pharma obtained rights for rare skin disease treatments. In October 2019, the US FDA approved AKLIEF (trifarotene) Cream, 0.005%, for the new retinoid molecule for the treatment of acne.
- AMZEEQ (minocycline): Journey Medical Corporation/Vyne Therapeutics Inc.
In 2019, the FDA approved AMZEEQ, a foam formulation of the tetracycline antibiotic minocycline, for treating severe acne in adults and children aged 9 years and older. AMZEEQ is specifically indicated for treating pimples and red bumps (non-nodular inflammatory lesions) linked to moderate to severe acne. This new formulation utilizes Foamix's proprietary Molecule Stabilizing Technology (MST) platform to deliver minocycline in a foam form, offering a more targeted and effective treatment option. Furthermore, minocycline is also approved by the FDA for treating rosacea in adult patients, expanding its therapeutic applications in dermatology. In January 2021, VYNE Therapeutics signed a contract with a major PBM to provide coverage for its topical minocycline products AMZEEQ and ZILXI, with the aim of expanding access for millions of patients with acne and rosacea.
- ARAZLO (tazarotene): Bausch Health Companies Inc. / Ortho Dermatologics
ARAZLO, a topical cream containing tazarotene as the active ingredient, is a treatment option for acne vulgaris in patients aged 9 years and older. The cream combines tazarotene with three moisturizers: sebacic acid, light mineral oil, and sorbitol, allowing it to be highly effective against acne while minimizing skin irritation. Additionally, ARAZLO lotion employs patented polymeric emulsion technology, which enhances skin absorption and reduces irritation, making it a comprehensive treatment option for acne patients.
Note: Detailed marketed therapies assessment will be provided in the final report.
Emerging Acne Vulgaris Drugs
- SB204: Pelthos Therapeutics
SB204, an investigational topical nitric oxide-releasing drug developed by Pelthos Therapeutics, has shown promising efficacy in treating acne vulgaris. This topical monotherapy is designed to address the multi-factorial nature of acne, which involves four key pathophysiological factors: inflammation, bacterial colonization, and other factors. SB204 utilizes the same active pharmaceutical ingredient as berdazimer gel 10.3% and is formulated to provide anti-inflammatory and anti-bacterial activity, making it a potential treatment option for acne patients.
B244: AOBiome LLC
B244 is a proprietary topical formulation that incorporates a single strain of beneficial AOB, Nitrosomonas eutropha, developed by AOBiome. This patented formulation is designed to restore the skin microbiome by repopulating it with AOBs that are naturally present in the body but often stripped away by most soaps. Once applied to the skin, B244 converts ammonia into nitrite, which exhibits antibacterial properties, and nitric oxide, a signaling molecule that regulates inflammation and vasodilation. Furthermore, this strain of AOB has been shown to reduce inflammatory and pruritic cytokines, such as IL-4, IL-5, IL-13, and IL-31, which are characteristic of atopic responses, thereby providing a comprehensive treatment option for skin conditions.
- DMT310: Dermata Therapeutics
DMT310, developed by Dermata Therapeutics from Spongilla platform technology, is under clinical investigation for the acne vulagris treatment. The novel product candidate comes from a naturally occurring source of Spongilla lacustris, which has several active ingredients that act by targeting interleukin 17A and 17F.
In November 2023, Dermata Therapeutics received an FDA response to its DMT310 Phase II study concentrating on the treatment of medical and cosmetic skin conditions. Currently the drug is being evaluated in Phase III of clinical development for the treatment of acne vulgaris.
|
Drug |
MoA |
RoA |
Company |
Logo |
Phase |
|
BPX-01 |
Glial cell inhibitors |
Topical |
Timber Pharmaceuticals/BioPharmX |
II | |
|
BTX 1503 |
Cannabinoid receptor CB1 inverse agonists |
Topical |
Botanix Pharmaceuticals |
II | |
|
XX |
XX |
X |
XXX |
X |
Acne Vulgaris Drugs Market Segmentation
DelveInsight’s ‘Acne Vulgaris Market Insights, Epidemiology, and Market Forecast – 2034’ report provides a detailed outlook of the current and future acne vulgaris drugs market, segmented within countries, by therapies, and by classes. Further, the market of each region is then segmented by each therapy to provide a detailed view of the current and future market share of all therapies.
Acne vulgaris Market Size by Countries
The Acne Vulgaris market size is assessed separately for various countries, including the United States, EU4 (Germany, France, Italy, and Spain), the UK, and Japan. In 2023, the United States held a significant share of the overall 7MM (Seven Major Markets) acne vulgaris drug market, primarily attributed to the country's higher prevalence of the condition and the elevated cost of the available treatments. This dominance is projected to persist, especially with the potential early introduction of new products. The acne vulgaris pipeline drugs market is evolving rapidly, with new clinical developments promising advanced treatments and improved patient outcomes.
Acne vulgaris Market Size by Therapies
Acne vulgaris Market Size by Therapies is categorized into current and emerging markets for the study period 2020–2034. One of the Emerging Acne Vulgaris Drugs anticipated to launch during the forecast period is BPX-01 under the developmental pipeline of BioPharmX.
Acne Vulgaris Market Outlook
Effective management of acne requires a comprehensive approach that addresses the underlying factors and incorporates lifestyle modifications, topical treatments, and systemic therapies as needed. The current Acne Vulgaris treatment market for acne vulgaris is driven by the increasing prevalence of acne, rising awareness of skincare, and the need for alternative treatment options.
Key developments include a diverse array of topical and systemic therapies, each targeting specific aspects of the complex pathogenesis of this common skin disorder. Topical treatments such as salicylic acid and azelaic acid are used to improve skin moisture, reduce inflammation, and eliminate comedones. Systemic therapies, including oral antibiotics like tetracycline and doxycycline, oral retinoids like isotretinoin, and hormonal agents like spironolactone, are utilized for severe cases.
Keeping in mind, that the current strategies are insufficient to manage the disease burden there is an urgent need for the development of novel therapy. The market awaits the launch of the potential entities SB204 (Pelthos Therapeutics), DMT310 (Dermata Therapeutics), B244 (AOBiome LLC.), BPX-01 (Timber Pharmaceuticals), BTX-1503 (Botanix Pharmaceuticals), and others that would be helpful in the reduction of affected population accomplishing a critical global public health need, if proved to be efficacious.
- The Acne Vulgaris Market Size in the 7MM was approximately USD 4,256 million in 2023 and is projected to increase during the forecast period (2024–2034).
- Among European countries, Germany accounted for the maximum Acne Vulgaris Market Size of USD 311 million in 2023 while the UK occupied the bottom of the ladder in the same year with USD 121 million.
- The Acne Vulgaris Market Size in Japan was nearly USD 316 million in 2023, which is further expected to increase by 2034.
Acne Vulgaris Drugs Uptake
This section focuses on the uptake rate of potential Acne Vulgaris drugs expected to launch in the Acne Vulgaris drugs market during 2020–2034. For example, DMT310 in the US is expected to be launched by 2026 with a peak share of 2%. DMT310 is anticipated to take 7 years to peak with medium-fast uptake. The acne medication market continues to grow with innovative treatments, addressing increasing demand for effective solutions to combat acne.
Acne Vulgaris Pipeline Development Activities
The Acne Vulgaris drugs market provides insights into different Acne Vulgaris clinical trials within Phase III, Phase II, and Phase I. It also analyzes key Acne Vulgaris Companies involved in developing targeted therapeutics.
Pipeline Development Activities
The Acne Vulgaris drugs market covers information on collaborations, acquisitions and mergers, licensing, and patent details for Acne Vulgaris emerging therapies.
KOL Views
To keep up with current market trends, we take KOLs and SMEs’ opinions working in the domain through primary research to fill the data gaps and validate the secondary research. Industry Experts were contacted for insights on Acne Vulgaris Treatment Market Landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake along with challenges related to accessibility, including KOL from the Department of Dermatology, Emory University School of Medicine, Atlanta, Georgia; Wake Forest University School of Medicine, North Carolina; Department of Dermatology and Venereology, University Clinic Hamburg-Eppendorf, Hamburg, Germany; La Roche-Posay Pharmaceutical Laboratory, France, and others.
Delveinsight’s analysts connected with 50+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. Their opinion helps understand and validate current and emerging therapies, treatment patterns, or Acne Vulgaris therapeutics market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving Acne Vulgaris treatment market landscape.
Conjoint Analysis analyzes multiple approved and emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the route of administration, order of entry and designation, probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Acne Vulgaris Therapeutics Market Access and Reimbursement
The high cost of therapies for the treatment is a major factor restraining the growth of the Acne Vulgaris drugs market. Because of the high cost, the economic burden is increasing, leading the patient to escape from proper treatment. The Acne Vulgaris drugs market report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of approved therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Acne Vulgaris Therapeutics Market Report Scope
- The Acne Vulgaris Therapeutics Market report covers a segment of key events, an executive summary, descriptive overview, explaining its causes, signs and symptoms, and currently available therapies.
- Comprehensive insight has been provided into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, disease progression, and treatment guidelines.
- Additionally, an all-inclusive account of the current and emerging therapies and the elaborative profiles of late-stage and prominent therapies will impact the current treatment landscape.
- A detailed review of the Acne Vulgaris Therapeutics Market, historical and forecasted Acne Vulgaris market size, market share by therapies, detailed assumptions, and rationale behind the approach is included in the report covering the 7MM drug outreach.
- The Acne Vulgaris Therapeutics Market Report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help shape and drive the 7MM Acne Vulgaris therapeutics Drugs Market.
Acne Vulgaris Therapeutics Market Report Insights
- Patient-based Acne Vulgaris Market Forecasting
- Therapeutic Approaches
- Acne Vulgaris Pipeline Analysis
- Acne Vulgaris Market Size and Trends
- Existing and Future Market Opportunity
Acne Vulgaris Therapeutics Market Report Key Strengths
- 11 years Acne Vulgaris Market Forecast
- The 7MM Coverage
- Acne Vulgaris Epidemiology Segmentation
- Key Cross Competition
- Conjoint Analysis
- Acne Vulgaris Drugs Market Uptake
- Key Acne Vulgaris Market Forecast Assumptions
Acne Vulgaris Therapeutics Market Report Assessment
- Current Acne Vulgaris Treatment Market Practices
- Acne Vulgaris Unmet Needs
- Acne Vulgaris Pipeline Product Profiles
- Acne Vulgaris Therapeutics Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
- Acne Vulgaris Market Drivers
- Acne Vulgaris Marjet Barriers
Key Questions Answered In The Acne Vulgaris Market Report:
Acne Vulgaris Treatment Market Insights
- What was the Acne Vulgaris market size, the market size by therapies, and Acne Vulgaris market share (%) distribution in 2020, and how it would all look in 2034? What are the contributing factors for this growth?
- Will the coverage of drugs depend on their efficacy in Acne Vulgaris?
- What will be the impact on the market with the launch of emerging drugs?
- Which is going to be the largest contributor to the market in 2034?
- What are the pricing variations among different geographies for approved and off-label therapies?
- How would the market drivers, barriers, and future opportunities affect the market dynamics and subsequent analysis of the associated trends?
Acne Vulgaris Epidemiology Insights
- What are the disease risks, burdens, and Acne Vulgaris Unmet Needs? What will be the growth opportunities across the 7MM with respect to the patient population pertaining to Acne Vulgaris?
- What is the historical and forecasted Acne Vulgaris patient pool in the United States, EU4 (Germany, France, Italy, and Spain) the United Kingdom, and Japan?
- Why do patients develop persistent Acne Vulgaris symptoms? Why is the current year diagnosis rate not high?
- Which type of severity is the largest contributor in patients affected with Acne Vulgaris?
- What factors are affecting the increase in the diagnosis of Acne Vulgaris?
Current Acne Vulgaris Treatment Market Scenario, Marketed Drugs, and Emerging Therapies
- What are the current options for the treatment of Acne Vulgaris? What are the current treatment guidelines for the treatment of Acne Vulgaris in the US and Europe?
- How many companies are developing therapies for the treatment of Acne Vulgaris?
- How many emerging therapies are in the mid-stage and late stage of development for the treatment of Acne Vulgaris?
- What are the recent novel therapies, targets, mechanisms of action, and technologies developed to overcome the limitations of existing therapies?
- What are the key designations that have been granted for the emerging therapies for Acne Vulgaris?
- What will be the impact on the market after the expected patent expiry of the emerging drug?
- What is the cost burden of off-label therapies on patients?
- Patient acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the country-specific accessibility issues of recommended therapies? Focus on reimbursement policies.
- What are the 7MM historical and forecasted Acne Vulgaris Market?
Reasons to Buy Acne Vulgaris Market Report:
- The Acne Vulgaris Treatment Market Report will help in developing business strategies by understanding the latest trends and changing treatment dynamics driving the Acne Vulgaris Drugs Market.
- Insights on patient burden/disease incidence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- To understand the existing market opportunity in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identification of strong upcoming players in the Acne Vulgaris Treatment Market will help in devising strategies that will help in getting ahead of competitors.
- Detailed analysis and potential of current and emerging therapies under the conjoint analysis section to provide visibility around leading emerging drugs.
- Highlights of Access and Reimbursement policies of approved therapies, barriers to accessibility of off-label expensive therapies, and patient assistance programs.
- To understand the perspective of Key Opinion Leaders around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the Acne Vulgaris unmet needs of the existing market so that the upcoming players can strengthen their development and launch strategy.
Read More Article:-