Agitation in Alzheimer’s Disease Market
- The Agitation in Alzheimer’s Disease market size is projected to experience a consistent CAGR during the forecast period (2024-2034), due to several factors. The key driver is an increase in the aging population, particularly in developed countries where the prevalence of Alzheimer's disease is higher. As the population ages, the number of individuals living with Alzheimer's disease and related symptoms, such as agitation, is expected to rise.
- Epidemiological data indicate that agitation is a common symptom in Alzheimer's disease patients, with prevalence varying across studies. The exact prevalence of agitation in Alzheimer's disease can be challenging to determine due to variations in diagnostic criteria and study methodologies. However, it's estimated that up to 50% of Alzheimer's patients experience symptoms of agitation at some point during their illness.
- Agitation in Alzheimer's disease presents a significant challenge for patients, with a lack of early detection, specific treatment, and curative therapy. REXULTI (brexpiprazole), is the first US FDA-approved treatment option for agitation associated with dementia due to Alzheimer’s disease.
- Several pharmaceutical companies are actively investigating and developing therapies for agitation in Alzheimer's disease. Johnson & Johnson, Otuska, IGC Pharma, Bioxcel, and others are among the leading companies in the industry, introducing novel and effective medicines to address the unmet requirements of Alzheimer's patients, particularly those suffering from agitation. Various emerging drugs, including AXS-05 (Axsome Therapeutics), AVP-786 (Otsuka Pharmaceutical), and IGC-AD1 (IGC Pharma), are anticipated to enter the market during the forecast period with the potential to influence the market dynamics.
Request for unlocking the CAGR of the "Agitation in Alzheimer’s Disease market"
DelveInsight’s comprehensive report titled “Agitation in Alzheimer’s Disease Market Insights, Epidemiology, and Market Forecast – 2034” offers a detailed analysis of Agitation in Alzheimer’s Disease. The report presents historical and projected epidemiological data covering total diagnosed prevalent cases of Alzheimer’s disease, total diagnosed prevalent cases of agitation in Alzheimer's disease, age-specific diagnosed prevalent cases of agitation in Alzheimer’s disease, gender-specific diagnosed prevalent cases of agitation in Alzheimer’s disease, severity-specific diagnosed prevalent cases of agitation in Alzheimer’s disease and treated cases of agitation in Alzheimer’s disease. In addition to epidemiology, the market report encompasses various aspects related to the patient population. These aspects include the diagnosis process, prescription patterns, physician perspectives, market accessibility, treatment options, and prospective developments in the market across seven major markets: the United States, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan, spanning from 2020 to 2034.
The report analyzes the existing treatment practices and unmet medical requirements in Agitation in Alzheimer’s Disease. It evaluates the market potential and identifies potential business prospects for enhancing therapies or interventions. This valuable information enables stakeholders to make well-informed decisions regarding product development and strategic planning for the market.
|
Study Period |
2020–2034 |
|
Forecast Period |
2024–2034 |
|
Geographies Covered |
US, EU4 (Germany, France, Italy, and Spain) and the UK, and Japan |
|
Agitation in Alzheimer’s Disease Epidemiology |
|
|
Agitation in Alzheimer’s Disease Market |
|
|
Market Analysis |
|
|
Agitation in Alzheimer’s Disease Market Players |
|
|
Challenges |
The existing treatments sometimes provide minimal relief, leaving a significant number of patients and caregivers burdened with ongoing agitation. This emphasizes the critical need for innovative medicines that can provide more powerful and prolonged relief of symptoms, providing further opportunities for market growth. |
Agitation in Alzheimer’s Disease Overview
Agitation in Alzheimer’s Disease Market may become agitated or aggressive as the disease gets worse. Agitation means that a person is restless or worried. The patient does not seem to be able to settle down. Agitation may cause pacing, sleeplessness, or aggression, which is when a person lashes out verbally or tries to hit or hurt someone. Therefore, Agitation in Alzheimer's disease is a common and distressing symptom characterized by disruptive behaviors in individuals.
Agitation in Alzheimer's Treatment Markert is multifactorial, arising from a combination of biological, psychological, and environmental factors. Neurobiological changes in the brain, including neurotransmitter imbalances and structural damage, contribute to behavioral disturbances. Comorbid conditions such as pain, infections, poor sleep, confusion, and medication side effects can exacerbate agitation. Agitation can also come from fear, such as believing they’re under threat.
Caregivers and the person with Alzheimer’s may need support to navigate changes that cause agitation and aggression. However, certain medications and therapeutic techniques can help.
Agitation in Alzheimer’s Treatment Market and Disease Diagnosis
The diagnosis of Agitation in Alzheimer's disease treatment market is usually based on clinical assessment, taking into account subjective reports from caregivers and observable behaviors in patients. These behaviors may include restlessness, pacing, verbal or physical aggression, and resistance to care. Healthcare professionals must conduct a thorough evaluation to rule out any underlying medical conditions or environmental factors contributing to the agitation. This may involve reviewing the patient's medical history, performing physical examinations, and sometimes utilizing imaging studies or laboratory tests. Additionally, standardized assessment scales such as the Cohen-Mansfield Agitation Inventory (CMAI) or the Neuropsychiatric Inventory (NPI) can provide valuable insights into the severity and frequency of agitation symptoms, aiding in treatment planning.
Agitation in Alzheimer’s Treatment Market often includes a multifaceted approach that addresses nonpharmacological and pharmacological interventions that often require the prescription of antipsychotics such as risperidone, quetiapine, and olanzapine is used to treat severe agitation, although use is associated with potential risks and, other similar drugs can be considered serotonin reuptake inhibitors (SSRIs) or psychotropic medications as alternatives based on individual patient characteristics and preferences require regular reassessment of patient symptoms and response to treatment to ensure appropriate management of aggression while minimizing potential harm.
Agitation in Alzheimer’s Disease Epidemiology
The epidemiology section on the Agitation in Alzheimer’s Disease treatment market report offers information on the patient populations, including historical and projected trends for each of the seven major markets. Examining key opinion leader views from physicians or clinical experts can assist in identifying the reasons behind historical and projected trends. The prevalent patient pool, their trends, and the underlying assumptions are all included in this section of the report.
This section also presents the data with relevant tables and graphs, offering a clear and concise view of the prevalence of Agitation in Alzheimer’s Disease. Additionally, the report discloses the assumptions made during the analysis, ensuring data interpretation and presentation transparency. This epidemiological data is valuable for understanding the disease burden and its impact on the patient population across various regions.
Agitation in Alzheimer’s Disease Key Findings
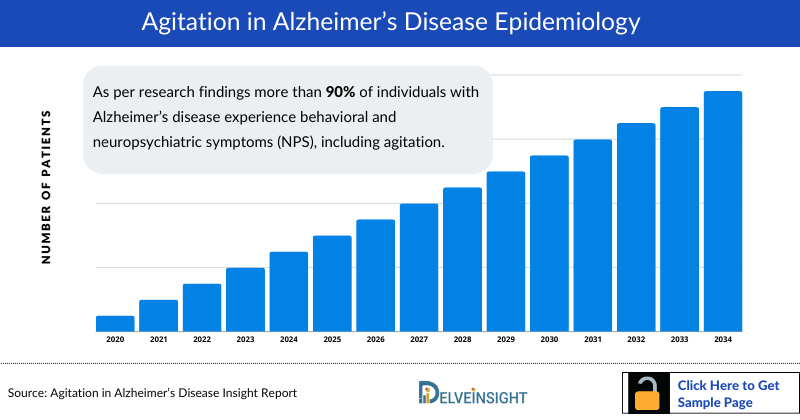
As per research findings more than 90% of individuals with Alzheimer’s disease experience behavioral and neuropsychiatric symptoms (NPS), including agitation.
At least 90% of patients with dementia experience behavioral or neuropsychiatric symptoms including agitation, psychotic symptoms, apathy, depression, and sleep disturbances. Therefore, agitation has been reported to be experienced by 60% of the patients with mild cognitive impairment and 76% of patients with Alzheimer’s disease.
In Europe, the range of prevalence of agitation was reported to be 24% to 88%, with 14 studies (47%) showing an agitation prevalence rate ≥50%.
A range of prevalence of agitation was reported as 23.3% to 78% in 10 studies, with two (17%) showing an agitation prevalence rate ≥50%.
Agitation in Alzheimer’s Disease Market Outlook
The therapeutic goals of the Agitation in Alzheimer’s Disease Market Size are based on controlling symptoms to improve patient well-being. Current treatment strategies often involve the use of antipsychotics such as risperidone or quetiapine, despite with increased risk associated with sedation, metabolism, and other adverse effects and stroke in elderly patients. Non-pharmacological interventions such as music therapy, aromatherapy, and routines can also play an important role in dealing with aggression, providing alternative methods of pharmacological treatment.
The majority of pharmacotherapies recommended for the treatment of Alzheimer’s disease include the use of dopamine and serotonin receptor antagonists that act by treating Alzheimer’s disease symptoms including agitation. Therefore, drugs and physical treatments should be included in the main sequential treatment algorithm for patients with Agitation in Alzheimer’s disease drug market.
Risperidone, quetiapine, aripiprazole, carbamazepine, citalopram, gabapentin, prazosin, trazodone, lorazepam, cyproterone acetate, and others are some of the commonly used pharmacotherapeutic treatment options. However, the efficacy of antipsychotic drugs comes at a cost for some individuals, as they carry risks of falls, excessive sedation, and metabolic abnormalities.
REXULTI (brexpiprazole) is the first US FDA-approved drug to treat agitation symptoms associated with dementia due to Alzheimer’s disease. REXULTI mediates through a combination of partial agonist activity at serotonin 5-HT1A and dopamine D2 receptors and antagonist activity at serotonin 5-HT2A receptors.
Agitation in Alzheimer's disease market requires a multidisciplinary approach involving neurologists, psychiatrists, nurses, social workers, and other healthcare professionals. There is a need for improved coordination and integration of care across different specialties to provide comprehensive and holistic management of agitation symptoms.
The Agitation in Alzheimer's disease market outlokk is changing, with novel medicines showing promise in addressing this challenging symptom. Among these are innovative pharmacological medicines that target specific neurotransmitter systems involved in agitation, such as serotonin antagonists, glutamate modulators, and drugs. These emerging drugs are designed to provide more tailored and effective symptom control while minimizing side effects.
Agitation in Alzheimer's Disease Recent Developments
- In March 2025, BioXcel Therapeutics, Inc. announced that the U.S. Food and Drug Administration (FDA) has closed the inspection of a single site in its TRANQUILITY II Phase 3 trial under 21 C.F.R.20.64(d)(3) and released the Establishment Inspection Report.
Agitation in Alzheimer's Disease Drug Chapters
Agitation in Alzheimer's Disease Drugs Market
REXULTI (brexpiprazole): Otsuka Pharmaceuticals
REXULTI, developed by Otsuka Pharmaceuticals, is the first US FDA-approved drug to treat agitation symptoms associated with dementia due to Alzheimer’s disease. The specific mechanism of action of REXULTI is not fully known, but it mediates through a combination of partial agonist activity at serotonin 5-HT1A and dopamine D2 receptors and antagonist activity at serotonin 5-HT2A receptors.
Note: Detailed marketed therapies assessment will be provided in the final report.
Emerging Agitation in Alzheimer’s Disease Drugs
The market for drugs targeting agitation in Alzheimer's Disease is witnessing rapid growth, driven by the increasing recognition of agitation as a major symptom requiring intervention and the emergence of innovative therapeutic candidates. Key market players, including Axsome, Otsuka, Johnson & Johnson and others have demonstrated a keen interest in this condition and are actively pursuing the development of potential treatments.
AXS-05: Axsome Therapeutics
AXS-05 is a novel, oral, investigational NMDA receptor antagonist, Cytochrome P-450 CYP2D6 inhibitor with multimodal activity being developed for the treatment of central nervous system conditions including Alzheimer's disease agitation. It consists of a proprietary formulation and dose of dextromethorphan (DM) and bupropion. The DM component of AXS-05 acts as an antagonist of the NMDA receptor, an ionotropic glutamate receptor, and a sigma-1 receptor agonist, these actions are thought to modulate glutamatergic neurotransmission. Whereas, the bupropion component of AXS-05 serves primarily to increase the bioavailability of dextromethorphan and is a norepinephrine and dopamine reuptake inhibitor. In 2022, the company reported, that AXS-05 achieved its primary endpoint in the ACCORD Phase III trial in Alzheimer’s disease agitation. Moreover, AXS-05 has been granted breakthrough therapy designation (BTD) by the US FDA for the treatment of Alzheimer’s disease agitation. Axsome received feedback from the US FDA on the clinical development program of AXS-05 in Alzheimer’s disease agitation. The FDA feedback indicated that an NDA for AXS-05 in agitation in Alzheimer’s disease should include placebo-controlled safety information from the ongoing Phase III, ADVANCE-2 trial; completion is slated for the first half of 2024. The company plans to apply for approval after this trial and its open-label extension are complete, in hopes of satisfying prior FDA feedback.
AVP-786: Otsuka Pharmaceutical Development & Commercialization
AVP-786 is an oral investigational therapeutic that works as an NMDA receptor antagonist/serotonin and norepinephrine reuptake inhibitor / sigma-1 receptor agonist in treating patients with agitation associated with dementia of Alzheimer's type. It is a deuterated, second-generation version of AVP-923/Nuedexta, a fixed-dose combination of two approved drugs. Deuterium is a naturally occurring, heavier isotope of hydrogen. Therefore, the combination of deudextromethorphan hydrobromide (d6-DM) and quinidine sulfate (Q), a CYP2D6 inhibitor is observed to significantly reduce susceptibility to cytochrome P450 (CYP2D6) enzyme metabolism thereby increasing the bioavailability. Recently the company announced topline results of the Phase III clinical trial of AVP-786 in the treatment of agitation associated with dementia due to Alzheimer’s disease.
A statistically significant difference was not achieved on the primary efficacy endpoint, mean change from baseline to Week 12 in the Cohen-Mansfield Agitation Inventory (CMAI) total score between AVP-786 and placebo. Full study results are not yet available. While the result of this trial was disappointing, the company plans to analyze the full data set to determine the future potential of AVP-786 in the treatment of agitation associated with dementia due to Alzheimer’s disease.
BXCL501: BioXcel Therapeutics
BXCL501 is an investigational proprietary, orally dissolving film formulation of dexmedetomidine, a selective alpha-2 adrenergic receptor agonist. It acts by potentially targeting an important mediator of agitation and has been observed as an anti-agitatant in multiple clinical studies across several neuropsychiatric disorders. BXCL501 is under investigation for the acute treatment of agitation associated with Alzheimer's dementia (at home). The company posted positive topline results from the TRANQUILITY II Phase III trial of BXCL501 for acute treatment of Alzheimer’s disease-related agitation. In addition, BXCL501 has been granted BTD for the acute treatment of agitation associated with dementia and Fast Track designation (FTD) for the acute treatment of agitation associated with schizophrenia, bipolar disorders, and dementia.
According to the last press release, the company was preparing for a meeting with the US FDA for its Phase III TRANQUILITY trial, which evaluated BXCL501 as a potential acute treatment of agitation associated with dementia caused by probable Alzheimer's disease in the home setting, with expected data in the first half of 2025.
Note: Detailed emerging therapies assessment will be provided in the final report.
Agitation in Alzheimer’s Disease Market Segmentation
DelveInsight’s ‘Agitation in Alzheimer’s Disease Market Insights, Epidemiology, and Market Forecast – 2034’ report provides a detailed outlook of the current and future Agitation in Alzheimer’s Disease market, segmented within countries, by therapies, and by classes. Further, the market of each region is then segmented by each therapy to provide a detailed view of the current and future market share of all therapies.
Agitation in Alzheimer’s Disease Market Size by Countries
The Agitation in Alzheimer’s Disease market size is assessed separately for various countries, including the United States, EU4 (Germany, France, Italy, and Spain), the UK, and Japan. In 2023, the United States held a significant share of the overall 7MM (Seven Major Markets) Agitation in the Alzheimer’s Disease market, primarily attributed to the country's higher prevalence of the condition and the elevated cost of the available treatments. This dominance is projected to persist, especially with the potential early introduction of new products.
Agitation in Alzheimer’s Disease Market Size by Therapies
Agitation in Alzheimer’s Disease Market Size by Therapies is categorized into current and emerging markets for the study period 2020–2034. One of the emerging drugs anticipated to launch during the forecast period is AXS-05 under the developmental pipeline of Axsome
Note: Detailed market segment assessment will be provided in the final report.
Agitation in Alzheimer’s Disease Drugs Uptake
This section focuses on the sales uptake of potential Agitation in Alzheimer’s Disease drugs that have recently been launched or are anticipated to be launched in the Agitation in Alzheimer’s Disease market between 2020 and 2034. It estimates the market penetration of Agitation in Alzheimer’s Disease drugs for a given country, examining their impact within and across classes and segments. It also touches upon the financial and regulatory decisions contributing to the probability of success (PoS) of the drugs in the Agitation in Alzheimer’s Disease market.
The emerging Agitation in Alzheimer’s Disease therapies is analyzed based on various attributes such as safety and efficacy in randomized clinical trials, order of entry, and other market dynamics, and the unmet need they fulfill in the Agitation in Alzheimer’s Disease market.
Note: Detailed assessment of drug uptake and attribute analysis will be provided in the full report on Agitation in Alzheimer’s Disease.
Agitation in Alzheimer’s Disease Market Access and Reimbursement
DelveInsight’s ‘Agitation in Alzheimer’s Disease – Market Insights, Epidemiology, and Market Forecast – 2034’ report provides a descriptive overview of the market access and reimbursement scenario of Agitation in Alzheimer’s Disease. This section includes a detailed analysis of the country-wise healthcare system for each therapy, enlightening the market access, reimbursement policies, and health technology assessments.
KOL Views
To keep up with current Agitation in Alzheimer’s Disease market trends and fill gaps in secondary findings, we interview KOLs and SMEs working in the Agitation in Alzheimer’s Disease domain. Their opinion helps understand and validate current and emerging therapies and treatment patterns or Agitation in Alzheimer’s Disease market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the Agitation in Alzheimer’s Disease unmet needs.
Agitation in Alzheimer’s Disease KOL Insights
DelveInsight’s analysts connected with 50+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. These KOLs were from organizations, institutes, and hospitals, such as the Hennepin County Medical Center in Minneapolis in the US, the Illinois Department of Public Health in the US, the Department of Psychiatry and Behavioral Neuroscience at St. Louis University School of Medicine in St. Louis, Missouri, and the and the School of Integrated Health Sciences at the University of Nevada, Las Vegas in the US, among others.
“Agitation in Alzheimer's disease manifests heterogeneously among individuals, making it challenging to develop standardized treatment approaches that address the diverse range of symptoms and underlying causes.”
“Limited healthcare resources, including access to specialized care, diagnostic tools, and support services, pose challenges to the effective management of agitation in Alzheimer's disease, particularly in underserved communities or regions with healthcare disparities.”
Note: Detailed assessment of KOL Views will be provided in the full report on Agitation in Alzheimer’s Disease.
Agitation in Alzheimer's Disease Competitive Intelligence Analysis
We conduct a Competitive and Market Intelligence analysis of the Agitation in Alzheimer’s Disease Market, utilizing various Competitive Intelligence tools such as SWOT analysis and Market entry strategies. The inclusion of these analyses is contingent upon data availability, ensuring a comprehensive and well-informed assessment of the market landscape and competitive dynamics.
Agitation in Alzheimer’s Disease Pipeline Development Activities
The report offers an analysis of therapeutic candidates in Phase II and III stages and examines companies involved in developing targeted therapeutics for Agitation in Alzheimer’s Disease. It provides valuable insights into the advancements and progress of potential treatments in clinical development for this condition.
Pipeline Development Activities
The report covers information on collaborations, acquisition and merger, licensing, patent details, and other information for emerging Agitation in Alzheimer’s Disease therapies.
Agitation in Alzheimer’s Disease Report Insights
- Agitation in Alzheimer’s Disease Patient Population
- Therapeutic Approaches
- Agitation in Alzheimer’s Disease Pipeline Analysis
- Agitation in Alzheimer’s Disease Market Size and Trends
- Agitation in Alzheimer’s Disease Market Opportunities
- Impact of Upcoming Therapies
Agitation in Alzheimer’s Disease Report Key Strengths
- 11 Years Forecast
- The 7MM Coverage
- Agitation in Alzheimer’s Disease Epidemiology Segmentation
- Key Cross Competition
- Highly Analyzed Agitation in Alzheimer’s Disease Market
- Agitation in Alzheimer’s Disease Drugs Uptake
Agitation in Alzheimer’s Disease Report Assessment
- Agitation in Alzheimer’s Disease Current Treatment Practices
- Unmet Needs
- Agitation in Alzheimer’s Disease Pipeline Product Profiles
- Agitation in Alzheimer’s Disease Market Attractiveness
Key Questions
- How common is Agitation in Alzheimer’s Disease?
- What are the key findings of Agitation in Alzheimer’s Disease epidemiology across the 7MM, and which country will have the highest number of patients during the study period (2020–2034)?
- What are the currently available treatments for Agitation in Alzheimer’s Disease?
- What are the disease risk, burden, and unmet needs of Agitation in Alzheimer’s Disease?
- At what CAGR is the Agitation in Alzheimer’s Disease market and its epidemiology expected to grow in the 7MM during the forecast period (2024–2034)?
- How would the unmet needs impact the Agitation in Alzheimer’s Disease market dynamics and subsequently influence the analysis of the related trends?
- What would be the forecasted patient pool of Agitation in Alzheimer’s Disease in the 7MM covering the United States, EU4 (Germany, France, Italy, and Spain), the UK, and Japan?
- Among EU4 and the UK, which country will have the highest number of patients during the forecast period (2020–2034)?
- How many companies are currently developing therapies for the treatment of Agitation in Alzheimer’s Disease?
Ready to Dive Deeper? Purchase the Complete Report for in-depth Market Analysis by Clicking Here @ Agitation in Alzheimer’s Disease Market Report
Access Exclusive Data Now! Click here to Read More about the Related Articles @ Latest DelveInsight Blog