Anaplastic Lymphoma Kinase Non Small Cell Lung Cancer Market Summary
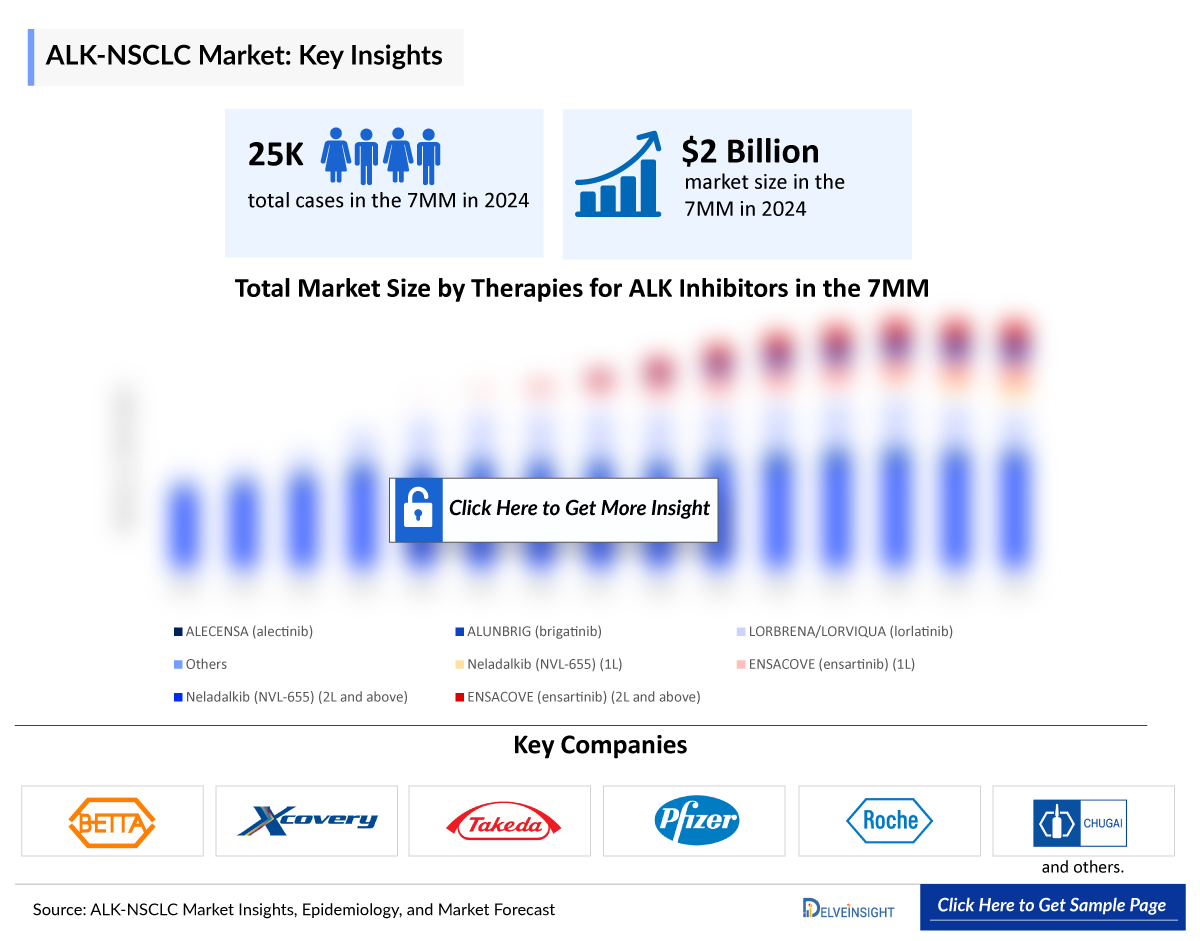
- The ALK NSCLC Market Size in the 7MM was approximately USD 2 billion in 2024 and is projected to increase during the forecast period (2025–2034).
- The leading ALK NSCLC Companies such as GlaxoSmithKline, Novartis, AstraZeneca, Pfizer Inc., F. Hoffmann-La Roche Ltd., Merck, Suzhou Zelgen Biopharmaceuticals Co.,Ltd, Ariad Pharmaceutica, Takeda, Chia Tai Tianqing Pharmaceutical Group, Akeso, and others.
ALK NSCLC Market and Epidemiology Analysis
- ALK NSCLC is a distinct molecular subtype of lung cancer characterized by ALK gene rearrangements that drive tumor growth and survival. This subgroup differs biologically and clinically from other NSCLC forms, underscoring the importance of molecular testing to identify patients eligible for targeted treatment approaches.
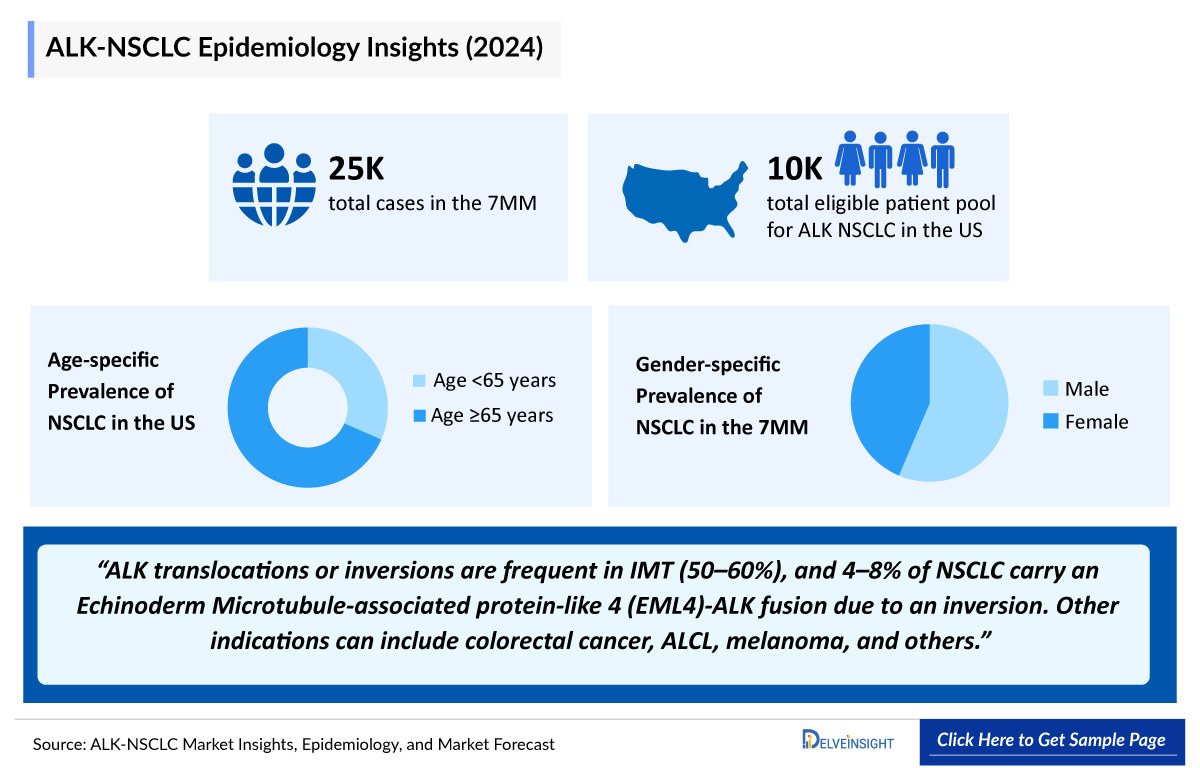
- In 2024, DelveInsight estimated that in the 7MM approximately 26,150 patients were considered as eligible ALK NSCLC patient pool, underscoring the significant clinical and commercial opportunity, the growing role of biomarker-driven patient identification, and the expanding adoption of targeted therapeutic approaches in this molecularly defined segment.
- The treatment landscape for ALK-positive cancers has significantly advanced with the availability of the US Food and Drug Administration (FDA)-approved targeted therapies, including ALECENSA (alectinib) by Roche, LORBRENA/LORVIQUA (lorlatinib) by Pfizer, ALUNBRIG (brigatinib) by Takeda, and ENSACOVE (ensartinib) by Betta Pharmaceuticals and Xcovery. These agents have transformed patient care by delivering durable responses, strong intracranial efficacy, and improved tolerability over conventional chemotherapy.
- The pipeline of ALK-positive cancers remains limited, with very few emerging therapies in development. Neladalkib (NVL-655), a fourth-generation ALK inhibitor by Nuvalent, is one of the few advancing candidates, while the lack of broader innovation leaves a clear gap in future treatment options.
Request for unlocking the Sample Page of the "ALK NSCLC Market"
Key Factors Driving the ALK-NSCLC Market
-
Targeted ALK NSCLC Patient Pool
In 2024, approximately 26K patients were considered as eligible patient pool for ALK NSCLC in 7MM, underscoring the significant clinical and commercial opportunity, the growing role of biomarker-driven patient identification, and the expanding adoption of targeted therapeutic approaches in this molecularly defined segment.
-
Advancements in Diagnostic Technologies
The shift towards personalized medicine, including genetic testing for ALK mutations, enhances the identification of patients who would benefit from ALK inhibitors. This trend is supported by ongoing advancements in diagnostic technologies, which facilitate timely treatment decisions.
-
Next-Generation ALK Inhibitors
ALK-targeted therapies, particularly next-generation inhibitors like alectinib and brigatinib, have shown significant improvements in progression-free survival (PFS) and overall survival (OS) compared to traditional chemotherapy for ALK-positive NSCLC patients.
DelveInsight’s “ALK NSCLC Market, Target Population, Competitive Landscape, and Market Forecast – 2034” report delivers an in-depth understanding of the ALK NSCLC, historical and projected epidemiological data, competitive landscape as well as ALK NSCLC market trends in the United States, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
The ALK NSCLC Market Report provides current treatment practices, emerging drugs, market share of individual therapies, and current and forecasted 7MM ALK NSCLC market size from 2020 to 2034. The report also covers emerging ALK NSCLC treatment practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s potential.
Scope of the ALK NSCLC Market | |
|
Study Period |
2020–2034 |
|
Forecast Period |
2025–2034 |
|
Geographies Covered |
US, EU4 (Germany, France, Italy, and Spain), the UK, and Japan |
|
ALK NSCLC Epidemiology |
Segmented by:
|
|
ALK NSCLC Companies |
|
|
ALK NSCLC Emerging Therapies |
|
|
ALK NSCLC Market |
Segmented By:
|
|
Analysis |
|
ALK NSCLC Disease Understanding and Treatment Algorithm
ALK NSCLC Overview
ALK NSCLC is a biologically distinct subtype of lung cancer driven by ALK gene rearrangements, which act as critical oncogenic drivers promoting tumor initiation and progression. This subset, though smaller within the overall NSCLC population, represents a clinically significant group due to its unique biology and responsiveness to targeted approaches. Advances in molecular diagnostics have improved identification of ALK-positive patients, while evolving treatment strategies have transformed the prognosis of this disease. Despite challenges such as resistance development and intracranial disease, ALK NSCLC remains a pivotal focus in lung cancer research and management, shaping both clinical outcomes and therapeutic innovation.
ALK NSCLC Treatment
ALK NSCLC treatment focuses on targeting the ALK rearrangement, a central driver of tumor growth and survival in this subtype of lung cancer. Therapies are designed to selectively inhibit ALK signaling, thereby suppressing uncontrolled cancer cell proliferation and halting disease progression. By blocking this oncogenic pathway, treatment strategies disrupt tumor development and achieve meaningful regression. In ALK NSCLC, modern therapies also address key challenges such as brain metastases and resistance mutations, providing a precision, disease-modifying approach. This has led to durable responses, prolonged survival, and improved clinical outcomes compared with conventional chemotherapy, marking a transformative shift in the management of this disease.
Further details related to disease overview are provided in the report…
ALK NSCLC Epidemiology
The ALK NSCLC epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented total incident cases of NSCLC, total eligible patient pool for ALK NSCLC, total incident cases of NSCLC by ALK biomarker positive and total treated cases of ALK NSCLC in the 7MM covering the United States, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan from 2020 to 2034.
Key Findings from ALK NSCLC Epidemiological Analyses and Forecast
- In 2024, DelveInsight estimated approximately 206,000 total incident cases of NSCLC in EU4 and the UK, underscoring the substantial disease burden in these regions and their critical role in shaping the clinical and therapeutic landscape of lung cancer.
- In 2024, DelveInsight estimated approximately 10,000 patients in the US comprising the total eligible patient pool for ALK NSCLC, emphasizing the significance of this subgroup in driving precision medicine uptake and targeted therapy adoption.
- In 2024, DelveInsight estimated approximately 4,400 total incident cases of NSCLC with positive ALK biomarker in Japan, highlighting the role of biomarker testing in identifying this clinically significant subgroup within the broader NSCLC population.
ALK NSCLC Epidemiology Segmentation
- Total ALK NSCLC Incident Cases
- Total ALK NSCLC Eligible Patient Pool
- Total Incident Cases of NSCLC by ALK Biomarker Positive
- Total ALK NSCLC Treated Cases
ALK NSCLC Drug Analysis
The drug chapter segment of the ALK NSCLC Therapeutics Market Reports encloses a detailed analysis of ALK NSCLC late-stage (Phase III and Phase I) and early stage ALK NSCLC pipeline drugs. It also helps understand the ALK NSCLC clinical trials details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug the latest news and press releases.
ALK NSCLC Marketed Drugs
-
ENSACOVE (ensartinib): Betta Pharmaceuticals/Xcovery
ENSACOVE (ensartinib) is an oral, small-molecule, next-generation ALK inhibitor being developed by Betta Pharmaceuticals/Xcovery to treat ALK NSCLC, including cases with brain metastases and resistance mutations.
-
- In December 2024, the US FDA approved ENSACOVE for adult patients with ALK-positive locally advanced or metastatic NSCLC who have not previously received an ALK inhibitor.
- In March 2024, the US FDA accepted the New Drug Application (NDA) for ensartinib for adult patients with metastatic ALK NSCLC, based on the Phase III eXalt3 study comparing its efficacy and safety to crizotinib in the 1L setting.
-
ALUNBRIG (brigatinib): Takeda
ALUNBRIG (brigatinib) by Takeda is a next-generation ALK inhibitor designed to improve outcomes for patients with ALK NSCLC. By selectively targeting ALK rearrangements, it inhibits tumor cell proliferation and survival, addressing resistance mutations and intracranial disease to enhance patient response and disease control. The therapy is approved for 1L, 2L and above in patients with ALK-positive advanced or metastatic NSCLC previously treated with crizotinib.
-
- In May 2020, Takeda reported that the US FDA approved ALUNBRIG for adult patients with ALK-positive metastatic NSCLC as detected by an FDA-approved test. This approval expands ALUNBRIG’s current indication to include the 1L setting.
- In April 2020, Takeda report that the EC extended the current marketing authorization of ALUNBRIG to include use as a monotherapy for the treatment of adult patients with ALK-positive advanced NSCLC previously not treated with an ALK inhibitor.
- In January 2021, Takeda obtained approval from the Japanese MHLW to manufacture and market ALUNBRIG tablets 30 mg and 90 mg as a 1L and 2L therapy for the treatment of patients with unresectable, advanced, or recurrent ALK fusion gene-positive NSCLC.
-
ALECENSA (alectinib): Roche
ALECENSA (alectinib) by Roche is a next-generation ALK inhibitor targeting ALK rearrangements, which drive tumor growth and survival in ALK NSCLC. The drug, already approved in multiple indications, has demonstrated potent systemic and intracranial activity, with a well-characterized safety profile, and is being further explored for expanded clinical applications. The therapy is approved across the US, Europe, and Japan for multiple treatment settings, including adjuvant, 1L, 2L and above therapy.
-
- In August 2024, Chugai Pharmaceutical reported that the MHLW had approved the additional indication of ALECENSA for adjuvant therapy for ALK fusion gene-positive NSCLC. The application for regulatory approval for ALECENSA was filed in December 2023.
- In June 2024, the European Commission (EC) approved ALECENSA monotherapy as adjuvant treatment following tumor resection for adult patients with ALK NSCLC at high risk of recurrence (Stage IB [=4 cm]–IIIA NSCLC).
- In April 2024, the US FDA approved ALECENSA for adjuvant treatment following tumor resection in patients with ALK NSCLC, as detected by an FDA-approved test.
List of ALK NSCLC Marketed Drugs | |||||
|
Product Name |
Company |
Approved Indications |
Initial Approval | ||
|
US |
EU |
JP | |||
|
ENSACOVE (ensartinib) |
Betta Pharmaceuticals/Xcovery |
1L: ALK+ NSCLC that have received up to 1 prior chemotherapy regimen and no prior ALK inhibitor. |
2024 |
- |
- |
|
ALUNBRIG (brigatinib) |
Takeda |
1L: Advanced ALK+ NSCLC who had not previously received an ALK-targeted therapy. 2L: ALK+ Advanced or metastatic NSCLC previously treated with crizotinib. |
2020, 2017 |
2020, 2018 |
2021 |
|
ALECENSA (alectinib) |
Roche/Chugai Pharmaceutical |
1L: ALK+ metastatic NSCLC. 2L and above: metastatic ALK+ NSCLC Previously Treated with Crizotinib |
2024, 2017, 2015 |
2024, 2017 |
2024, 2014 |
|
XX |
XX |
XX |
XX |
XX |
XX |
ALK NSCLC Emerging Drugs
-
Neladalkib (NVL-655): Nuvalent
Neladalkib (NVL-655) is an oral, small-molecule, fourth-generation ALK inhibitor being developed by Nuvalent to treat ALK NSCLC, including patients with tumors harboring resistance mutations or brain metastases. Designed to overcome limitations of earlier-generation ALK inhibitors, neladalkib exhibits potent systemic and intracranial activity, aiming to provide durable responses in patients with advanced ALK-driven cancers. The drug is under evaluation in two trials: the Phase III ALKAZAR study in 1L treatment and the Phase I/II ALKOVE-1 trial in 2L and above settings, with pivotal topline results from ALKOVE-1 in TKI-pretreated ALK+ NSCLC expected by late 2025.
-
- In July 2025, Nuvalent reported the initiation of the ALKAZAR Phase III randomized, controlled trial evaluating neladalkib (NVL-655) in patients with TKI-naïve ALK NSCLC
- In May 2024, Nuvalent reported that the US FDA granted Breakthrough Therapy Designation (BTD) to NVL-655 for patients with locally advanced or metastatic ALK NSCLC previously treated with two or more ALK TKIs, based on early clinical evidence of safety and efficacy. The FDA also awarded Orphan Drug Designation (ODD) for NVL-655 in ALK NSCLC.
List of ALK NSCLC Emerging Drugs | ||||
|
Drug Name |
Company |
Indication |
Mechanism of Action |
Highest Phase |
|
Neladalkib (NVL-655) |
Nuvalent |
Other solid tumors harboring an ALK rearrangement or activating ALK mutation, who have received ≥1 prior systemic anticancer therapy, or for whom no satisfactory standard therapy exists. |
ALK inhibitors |
Phase III |
|
XX |
XX |
XX |
XX |
XX |
ALK NSCLC Market Outlook
The ALK NSCLC Market Landscape is expected to expand significantly in the coming years, driven by the increasing incidence of NSCLC, growing awareness of the clinical benefits of targeted therapy, the emergence of next- and fourth-generation ALK inhibitors, and continued investment from major pharmaceutical companies.
ALK NSCLC represents a transformative class of therapies that selectively target ALK rearrangements, a key driver of tumor growth and survival. In ALK NSCLC, these agents not only suppress tumor proliferation but also demonstrate potent intracranial activity, addressing the high unmet need in patients with brain metastases and resistance mutations. Emerging compounds, such as neladalkib (NVL-655), are designed to overcome limitations of earlier-generation therapies, offering durable systemic and intracranial responses even in heavily pretreated patients.
By targeting the oncogenic driver at the molecular level, ALK inhibitors provide precision, disease-modifying benefits compared with conventional chemotherapy, improving outcomes and quality of life for patients with ALK-driven cancers. Ongoing clinical studies and regulatory milestones will continue to expand treatment options and solidify the role of ALK inhibitors as a cornerstone in the management of ALK-positive malignancies.
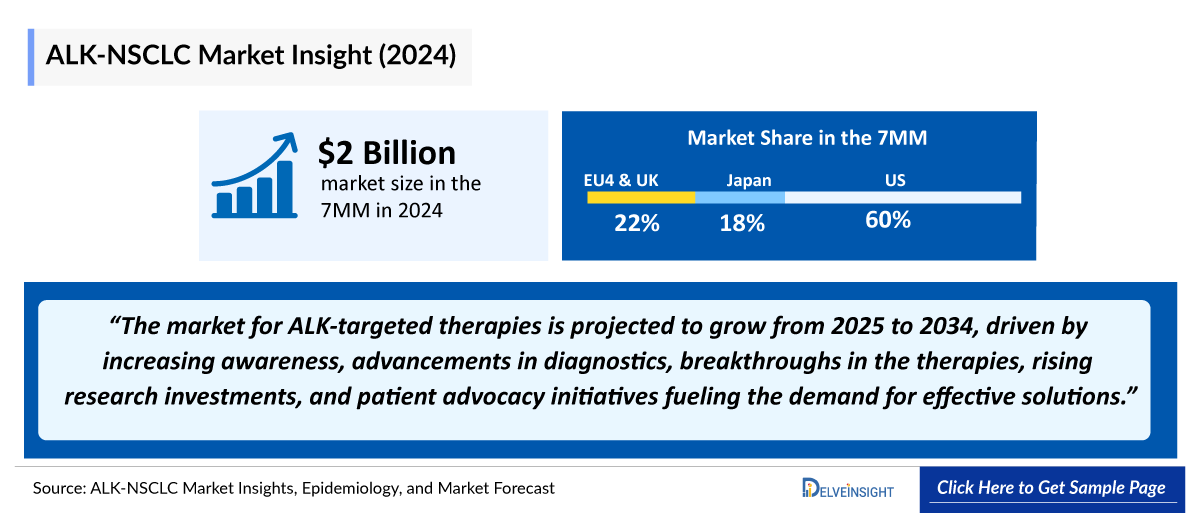
- The ALK NSCLC Market Size in the 7MM was approximately USD 2 billion in 2024 and is projected to increase during the forecast period (2025–2034).
- The ALK NSCLC Market Size in the US was more than USD 1.2 billion in 2024 and is anticipated to increase due to the launch of emerging therapies.
- The ALK NSCLC Market Size of EU4 and the UK was calculated to be approximately USD 440 million in 2024, which was nearly 22% of the total market revenue for the 7MM and is expected to increase by 2034.
- In 2024, Germany dominated the market between EU4 and the UK, generating more than USD 115 million. The UK followed closely with around USD 95 million, while Spain recorded the least.
- In 2024, the ALK NSCLC Market Size was approximately USD 330 million in Japan, which is anticipated to increase during the forecast period (2025-2034).
ALK NSCLC Drugs Uptake
This section focuses on the uptake rate of potential emerging ALK NSCLC expected to be launched in the market during 2020–2034.
ALK NSCLC Pipeline Development Activities
The ALK NSCLC Therapeutics Market Report provides insights into different therapeutic candidates in preregistration, Phase III, and early stage molecule. It also analyzes key ALK NSCLC Companies involved in developing targeted therapeutics. The presence of numerous drugs under different stages is expected to generate immense opportunity for ALK NSCLC market growth over the forecasted period. The report covers information on collaborations, acquisitions and mergers, licensing, and patent details for ALK NSCLC emerging therapies.
Latest KOL Views on ALK NSCLC
To keep up with current and future market trends, we take industry experts’ opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Industry experts were contacted for insights on ALK NSCLC evolving ALK NSCLC Treatment Market Landscape, patient reliance on conventional therapies, patient therapy switching acceptability, drug uptake, along challenges related to accessibility. DelveInsight’s analysts connected with 25+ KOLs to gather insights; however, interviews were conducted with 10+ KOLs in the 7MM. Centers like the Dana-Farber Cancer Institute, US; University Hospital Heidelberg, Germany; Comprehensive Cancer Center, France; San Raffaele Scientific Institute, Italy; La Paz University Hospital, Spain; Royal Marsden Hospital, United Kingdom; and Tokyo Medical University, Japan, among others. Their opinion helps understand and validate current and emerging therapy treatment patterns or ALK NSCLC market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
Physician’s View
As per KOL from the US, “ALK-positive lung cancer represents a small subset of NSCLC, and guidelines recommend routine testing in advanced nonsquamous cases. ALK inhibitors have transformed outcomes, with adjuvant alectinib, with or without chemotherapy, now also endorsed in resected early-stage disease.” As per KOL from the UK, “Brigatinib not only shows superior efficacy and tolerability over crizotinib but also signals a potential survival advantage in patients with brain metastases, underscoring its ability to address both systemic and CNS disease, which remains a critical challenge in ALK NSCLC. This highlights the importance of CNS-active therapies in shaping future treatment strategies.”
As per KOL from Japan, “CNS metastases and acquired resistance remain major challenges in ALK-positive advanced NSCLC. Network meta-analyses demonstrate that third-generation ALK inhibitors provide the greatest benefit in prolonging progression-free survival, although differences in CNS-active efficacy between individual agents may be less pronounced. These findings underscore the importance of generation- and drug-specific selection in developing individualized treatment strategies and support ongoing translational and clinical research to optimize outcomes in patients with CNS involvement.”
ALK NSCLC Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
ALK NSCLC Market Access and Reimbursement
Reimbursement for ALK NSCLC is well established in the United States and is steadily expanding across Europe. Manufacturers offer patient assistance programs that provide free or discounted access for eligible uninsured or underinsured patients. While these programs can significantly reduce out-of-pocket costs, patients remain responsible for co-pays, deductibles, and clinic visit fees. Coverage typically excludes claims reimbursed by Medicaid, Medicare, or other federal or state healthcare programs. In the US, programs for ALECENSA (alectinib) include the Genentech Oncology Co-pay Assistance Program, independent co-pay assistance foundations, and the Genentech Patient Foundation, helping eligible patients access therapy at reduced or no cost.
The report further details country-wise reimbursement and accessibility status, cost-effectiveness assessments, patient assistance initiatives that improve affordability, and insights into coverage under government prescription drug programs.
Scope of the ALK NSCLC Market Report
- The ALK NSCLC Market report covers a segment of key events, an executive summary, and a descriptive overview, explaining their mechanism and therapies (current and emerging).
- Comprehensive insight into the competitive landscape, and forecasts, the future growth potential of treatment rate, drug uptake, and drug information have been provided.
- Additionally, an all-inclusive account of the current and emerging therapies and the elaborative profiles of late-stage and prominent therapies will impact the current ALK NSCLC market landscape.
- A detailed review of the ALK NSCLC Market, historical and forecasted ALK NSCLC Market Size, ALK NSCLC Market Share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The ALK NSCLC Market Report provides an edge while developing business strategies, by understanding trends, through SWOT analysis, expert insights/KOL views, and treatment preferences that help shape and drive the 7MM ALK NSCLC Market.
ALK NSCLC Market Report Insights
- ALK NSCLC Targeted Patient Pool
- ALK NSCLC Therapeutic Approaches
- ALK NSCLC Pipeline Analysis
- ALK NSCLC Market Size and Trends
- Existing and Future ALK NSCLC Market Opportunity
ALK NSCLC Market Report Key Strengths
- 10 years ALK NSCLC Market Forecast
- The 7MM Coverage
- Key Cross Competition
- Attribute Analysis
- ALK NSCLC Drugs Uptake
- Key ALK NSCLC Market Forecast Assumptions
ALK NSCLC Market Report Assessment
- Current ALK NSCLC Treatment Practices
- ALK NSCLC Unmet Needs
- ALK NSCLC Pipeline Drugs Profiles
- ALK NSCLC Market Attractiveness
- ALK NSCLC Qualitative Analysis (SWOT)
Key Questions Answered in the ALK NSCLC Market Report
ALK NSCLC Market Insights
- What was the ALK NSCLC Market Size, the ALK NSCLC Market Size by therapies, ALK NSCLC Market Share (%) distribution in 2024, and what would it look like in 2034? What are the contributing factors for this growth?
- Which drug is going to be the largest contributor in 2034?
- Which is the most lucrative ALK NSCLC Market?
- Which drug accounts for maximum ALK NSCLC sales?
- What are the risks, burdens, and unmet needs of treatment with ALK NSCLC? What will be the growth opportunities across the 7MM for the patient population ALK NSCLC?
- What are the key factors hampering the growth of the ALK NSCLC Market?
- What are the indications for which recent novel therapies and technologies have been developed to overcome the limitations of existing treatments?
- What key designations have been granted to the therapies for ALK NSCLC?
- Patient acceptability in terms of preferred therapy options as per real-world scenarios?
Reasons to Buy the ALK NSCLC Market Report
- The ALK NSCLC Market Report will help develop business strategies by understanding the latest trends and changing dynamics driving the ALK NSCLC Market.
- Understand the existing ALK NSCLC Market opportunities in varying geographies and the growth potential over the coming years.
- Identifying strong upcoming players in the market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of indication-wise current and emerging therapies under the attribute analysis section to provide visibility around leading indications.
- To understand key opinion leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing ALK NSCLC Market so that the upcoming players can strengthen their development and launch strategy.
Stay updated with us for Recent Articles