Bacterial Vaginosis Market
- The Bacterial Vaginosis Market size is anticipated to sustain a steady Compound Annual Growth Rate (CAGR) from 2024 to 2034. This growth in market revenue is chiefly propelled by advancements in diagnostic techniques, heightened awareness of the condition, and a growing number of reported cases.
- The increase in bacterial vaginosis cases can be attributed to several factors. Poor hygiene practices, such as the misuse of hygiene products or inadequate cleansing routines, create an environment conducive to bacterial overgrowth. Engaging in sexual activity with multiple partners or inconsistent condom usage increases the risk of exposure to bacterial pathogens. Additionally, factors such as hormonal fluctuations, douching, and certain medical conditions can disrupt the balance of vaginal bacteria, predisposing individuals to bacterial vaginosis.
- Bacterial Vaginosis companies are advancing treatment options through innovative research and drug development. Key Bacterial Vaginosis players include Aurobindo Pharma, AbbVie Inc., Bayer AG, Melinta Therapeutics, Inc, Bristol-Myers Squibb Company, GlaxoSmithKline plc., Lupin, Osel, Darebioscience, Organon, Gedea Biotech, and others.
- The existing bacterial vaginosis market is characterized by high competition with already established key players including Organon, Daré Bioscience, Lupin, and others.
- Current treatments of bacterial vaginosis are associated with unacceptably high recurrence factors. Clinicians and researchers in this field consider an urgent priority to develop new and innovative approaches for tailoring the management of bacterial vaginosis to achieve high and sustained long-term cure rates.
- To accomplish and meet the unmet needs the future of bacterial vaginosis treatment is continuing to incline toward combined topical and oral antimicrobial therapies as well as more novel methods including approaches that aim to restore a woman’s “healthy” vaginal microbiome and maintain homeostasis are much needed to prevent recurrent bacterial vaginosis and its sequela, including transmission and acquisition of HIV and adverse obstetric outcomes, such as preterm birth.
- To drive the bacterial vaginosis market in future years, several Bacterial Vaginosis Companies such as Osel, and others are developing their assets in the mid-late stage of development. With the expected approval of these therapies during the forecast period [2024–2034], the overall Bacterial Vaginosis Market Size is likely to witness a rise at a significant CAGR.
Request for unlocking the CAGR of the "Bacterial Vaginosis Drug Market"
DelveInsight’s comprehensive report titled “Bacterial Vaginosis Market Insights, Epidemiology, and Market Forecast – 2034” offers a detailed analysis of bacterial vaginosis. The report presents historical and projected epidemiological data covering Total Prevalent Cases and Diagnosed Prevalent Cases of Bacterial vaginosis further segmented by Age. In addition to epidemiology, the market report encompasses various aspects related to the patient population. These aspects include the diagnosis process, prescription patterns, physician perspectives, market accessibility, treatment options, and prospective developments in the Bacterial Vaginosis therapeutics market across seven major markets: the United States, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan, spanning from 2020 to 2034.
The report analyzes the existing Bacterial Vaginosis treatment market practices and unmet medical requirements in bacterial vaginosis. It evaluates the market potential and identifies potential business prospects for enhancing therapies or interventions. This valuable information enables stakeholders to make well-informed decisions regarding product development and strategic planning for the market.
|
Study Period |
2020 to 2034 |
|
Forecast Period |
2024-2034 |
|
Geographies Covered |
|
|
Bacterial vaginosis Market |
|
|
Bacterial vaginosiss Market Size | |
|
Bacterial vaginosis Companies |
Aurobindo Pharma, AbbVie Inc., Bayer AG, Melinta Therapeutics, Inc, Bristol-Myers Squibb Company, GlaxoSmithKline plc., Lupin, Osel, Darebioscience, Organon, Gedea Biotech, Pfizer Inc, Mission Pharmacal Company, Siolta Therapeutics, Mylan N.V., Novartis AG, Hikma Pharmaceuticals plc, and others. |
|
Bacterial vaginosis Epidemiology Segmentation |
|
Bacterial Vaginosis Treatment Market
Bacterial vaginosis also referred to as nonspecific vaginitis, is a vaginal condition that can produce vaginal discharge and results from an overgrowth of certain kinds of bacteria in the vagina. Bacterial vaginosis is the most common infectious vaginitis. The exact cause of bacterial vaginosis is unknown. There are several bacteria associated with it. Bacterial vaginosis can occur from an overgrowth of pathogens such as Gardnerella spp., Prevotella spp., Mobilincus spp., Megaspahera spp., Sneathea spp., and mixed vaginal anaerobic species, with many species facilitating growth and replacing the beneficial lactobacilli that help maintain a healthy vaginal environment. Ongoing Bacterial Vaginosis clinical trials are exploring innovative antibiotics and probiotics aimed at reducing recurrence rates and improving long-term outcomes, offering hope for more effective and lasting treatment options.
Bbacterial vaginosis Risk Factors are the same as those for sexually transmitted infections. Certain risk factors have been identified that increase the chances of developing Bacterial Vaginosis. These risk factors for bacterial vaginosis include antibiotic use, intrauterine devices for birth control, and cigarette smoking. Bacterial vaginosis appears to increase the risk of pelvic inflammatory disease, post abortion and postpartum endometritis, and post hysterectomy vaginal cuff infection. In pregnancy, bacterial vaginosis is associated with an increased risk of chorioamnionitis, premature rupture of membranes, preterm labor, and preterm birth.
Many women with Bacterial Vaginosis have no signs or symptoms at all. When symptoms do occur, the most common include, an abnormal amount of vaginal discharge, thin and grayish white vaginal discharge and vaginal odor. Symptoms of Bacterial Vaginosis, if present, can occur any time in the menstrual cycle, including before, during, or after the menstrual period.
Bacterial Vaginosis Diagnosis and Treatment Algorithm
A diagnosis of bacterial vaginosis includes a pelvic examination, vaginal pH, and microscopy. If microscopy is unavailable, sometimes nucleic acid amplification tests (NAATs) are considerable. For bacterial vaginosis to be diagnosed, 3 of 4 criteria (Amsel criteria) must be present, including Yellow-green or gray discharge, Vaginal secretion pH > 4.5, Fishy odor on the whiff test (application of potassium hydroxide), and Clue cells on saline (0.9%) wet mount.
Currently, the major treatment options for Bacterial Vaginosis are metronidazole, clindamycin oral or vaginal suppositories, and metronidazole vaginal gel. Although up to 30% of bacterial vaginosis cases may resolve without treatment, this condition can also be treated with either clindamycin or metronidazole. Both of these medications are effective if taken by mouth or applied vaginally. Additionally, both are safe to be used by pregnant women.
Bacterial Vaginosis Epidemiology
The Bacterial vaginosis epidemiology section on the Bacterial vaginosis drug market report offers information on the patient populations, including historical and projected trends for each of the seven major markets. Examining key opinion leader views from physicians or clinical experts can assist in identifying the reasons behind historical and projected trends. The diagnosed patient pool, their trends, and the underlying assumptions are all included in this section of the report.
This section also presents the data with relevant tables and graphs, offering a clear and concise view of the prevalence of bacterial vaginosis. Additionally, the Bacterial Vaginosis drug market report discloses the assumptions made during the analysis, ensuring data interpretation and presentation transparency. This epidemiological data is valuable for understanding the disease burden and its impact on the patient population across various regions.
Key Findings
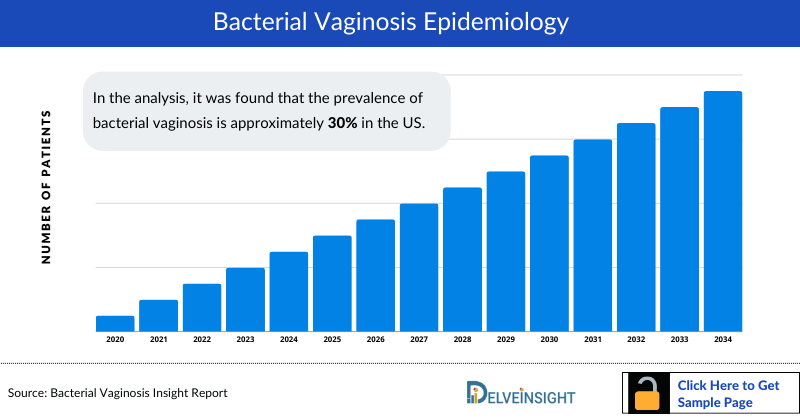
- During the bacterial vaginosis analysis, it was found that bacterial vaginosis is the most common vaginal infection found in women of reproductive age. It is estimated to occur in anywhere 5–70% of women.
- In the analysis, it was found that the prevalence of bacterial vaginosis is approximately 30% in the US.
- During the analysis, bacterial vaginosis was found to occur in 14–49 years women in women. However, rates are variable between different ethnic groups and are most common in non-white women.
- It was found that the Bacterial Vaginosis prevalence is notable even among women who have not engaged in vaginal, oral, or anal sex, affecting 18.8% of this population.
- The Bacterial vaginosis epidemiology is expected to change during the forecast period (2024-2034).
Bacterial vaginosis Market Outlook
The bacterial vaginosis market outlook is further expected to increase by the major drivers, such as the rising prevalent population, technological advancements, and upcoming therapies in the forecast period [2024–2034]. In December 2021, Daré announced that the US FDA has approved XACIATO (clindamycin phosphate) vaginal gel, 2% (formerly known as DARE-BV1) for the treatment of bacterial vaginosis in females 12 years of age and older. In March 2022, Organon and Daré Bioscience, Inc. announced that they have entered into an agreement whereby Organon will license global rights to XACIATO (clindamycin phosphate) vaginal gel, 2%.
Another approved therapy is VIVAGEL BV, formulated as a mucoadhesive gel, which was approved in Europe in the year 2019. It is the first non-antibiotic bacterial vaginosis treatment for the prevention of recurrent bacterial vaginosis. As a result, the product has both QIDP and Fast Track status for both indications covering other markets. VIVAGEL BV is available for sale under the brand names BETAFEM BV Gel (UK), and BETADINE BVTM (Europe). With ongoing research and continued dedication, the future holds hope for even more effective treatments and, ultimately, a cure for this challenging condition. According to DelveInsight, the Bacterial vaginosis market in the 7MM is expected to change significantly during the study period 2020–2034.
Bacterial Vaginosis Recent Developments
- In August 2025, Evofem Biosciences, Inc. (OTCID: EVFM) announced that a newly issued U.S. patent covering SOLOSEC® (secnidazole) 2 g oral granules and one of its labeled indications has been listed in the U.S. Food and Drug Administration (FDA) publication Approved Drug Products with Therapeutic Equivalence Evaluations (the Orange Book).
Bacterial Vaginosis Drug Chapters
The Bacterial Vaginosis drugs market is experiencing steady growth, driven by increasing awareness, improved diagnostics, and rising demand for effective treatments targeting recurring infections and antibiotic-resistant bacterial strains.
Bacterial Vaginosis Marketed Drugs
- XACIATO: Organon/Daré Bioscience
XACIATO, is a colorless single-dose vaginal gel that can be applied at any time of day and is formulated to limit leakage and increase vaginal retention time (time spent in place). As demonstrated by an in vitro study using clindamycin HCl, the gel increases viscosity (thickness and stickiness) at body temperature and gradually releases clindamycin, over time. XACIATO is indicated for the treatment of bacterial vaginosis in females 12 years and older. A single-dose user-filled disposable applicator delivers 5g of vaginal gel containing 100mg of clindamycin.
- SOLOSEC: Lupin
SOLOSEC (secnidazole) 2g oral granules is the first and only single-dose oral prescription approved to treat both bacterial vaginosis, a common vaginal infection, in female patients 12 years of age and older. SOLOSEC is designed to be easy to take and one oral dose contains a complete course of treatment.
Note: Detailed marketed therapies assessment will be provided in the final report.
Bacterial Vaginosis Emerging Drugs
The Bacterial vaginosis drugs market outlook is expected to experience gradual changes, mainly due to the limited availability of emerging therapies in this area. Key Bacterial Vaginosis Companies, including Osel, and others, have demonstrated a keen interest in this condition and are actively pursuing the development of potential treatments.
- LACTIN-V: Osel
Osel’s LACTIN-V is a live bio therapeutic product addressing women’s health disorders. LACTIN-V is under development for preventing recurrent bacterial vaginosis. It contains Lactobacillus crispatus CTV-05, a single strain of hydrogen peroxide-producing vaginal Lactobacillus that is part of the natural vaginal microbiome of many healthy women. LACTIN-V reduces recurrent bacterial vaginosis in women at high risk by promoting the colonization of L. crispatus. LACTIN-V is delivered vaginally with a proprietary applicator. LACTIN-V is currently in Phase II of clinical development.
Note: Detailed emerging therapies assessment will be provided in the final report.
Bacterial Vaginosis Market Segmentation
DelveInsight’s ‘Bacterial vaginosis Drug Market Insights, Epidemiology, and Market Forecast – 2034’ report provides a detailed outlook of the current and future Bacterial vaginosis drug market, segmented within countries, by therapies, and by classes. Further, the market of each region is then segmented by each therapy to provide a detailed view of the current and future market share of all therapies.
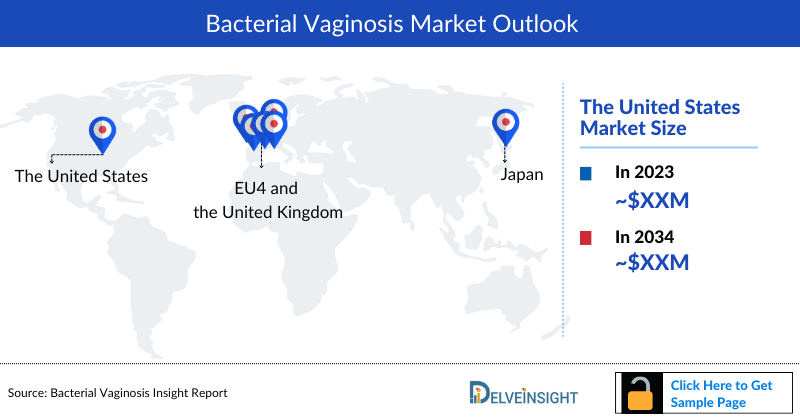
Bacterial vaginosis Market Size by Countries
The Bacterial vaginosis market size is assessed separately for various countries, including the United States, EU4 (Germany, France, Italy, and Spain), the UK, and Japan. In 2023, the United States held a significant share of the overall 7MM (Seven Major Markets) Bacterial vaginosis market, primarily attributed to the country's higher prevalence of the condition and the elevated cost of the available treatments. This dominance is projected to persist, especially with the potential early introduction of new products.
Bacterial vaginosis Market Size by Therapies
Bacterial vaginosis Market Size by therapies is categorized into current and emerging markets for the study period 2020–2034. One of the emerging drugs anticipated to launch during the forecast period is LACTIN-V under the developmental pipeline of Osel.
Note: Detailed market segment assessment will be provided in the final report.
Bacterial vaginosis Drugs Uptake
This section focuses on the sales uptake of potential bacterial vaginosis drugs that have recently been launched or are anticipated to be launched in the bacterial vaginosis market between 2020 and 2034. It estimates the market penetration of Bacterial vaginosis drugs for a given country, examining their impact within and across classes and segments. It also touches upon the financial and regulatory decisions contributing to the probability of success (PoS) of the drugs in the bacterial vaginosis market.
The emerging bacterial vaginosis therapies are analyzed based on various attributes such as safety and efficacy in randomized clinical trials, order of entry and other market dynamics, and the unmet need they fulfill in the Bacterial vaginosis market.
Note: Detailed assessment of drug uptake and attribute analysis will be provided in the full report on bacterial vaginosis.
Bacterial vaginosis Market Access and Reimbursement
DelveInsight’s ‘Bacterial vaginosis Market Insights, Epidemiology, and Market Forecast – 2034’ report provides a descriptive overview of the market access and reimbursement scenario of bacterial vaginosis. This section includes a detailed analysis of the country-wise healthcare system for each therapy, enlightening the market access, reimbursement policies, and health technology assessments.
KOL Views
To keep up with current Bacterial vaginosis market trends and fill gaps in secondary findings, we interview KOLs and SMEs’ working in the bacterial vaginosis domain. Their opinion helps understand and validate current and emerging therapies and treatment patterns or Bacterial vaginosis market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the Bacterial vaginosis unmet needs.
Bacterial vaginosis: KOL Insights
DelveInsight’s analysts connected with 50+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. These KOLs were from organizations, institutes, and hospitals, such as the Division of Infectious Diseases, University of Alabama at Birmingham, Birmingham, AL USA, Division of Infectious Diseases, Wayne State University, Detroit, MI USA, Department of Obstetrics and Gynecology, University of Washington, Seattle, among others.
“It is common for bacterial vaginosis to recur within three to 12 months, despite treatment. Researchers are exploring treatments for recurrent bacterial vaginosis”
Note: Detailed assessment of KOL Views will be provided in the full report on bacterial vaginosis.
Competitive Intelligence Analysis
We conduct a Competitive and Market Intelligence analysis of the Bacterial vaginosis Market, utilizing various Competitive Intelligence tools such as SWOT analysis and Market entry strategies. The inclusion of these analyses is contingent upon data availability, ensuring a comprehensive and well-informed assessment of the market landscape and competitive dynamics.
Bacterial Vaginosis Pipeline Development Activities
The Bacterial vaginosis pipeline report offers an analysis of Bacterial vaginosis clinical trials within Phase II and III stages and examines Bacterial vaginosis companies involved in developing targeted therapeutics for bacterial vaginosis. It provides valuable insights into the advancements and progress of potential Bacterial vaginosis treatments in clinical development for this condition.
Bacterial vaginosis Pipeline Development Activities
The Bacterial vaginosis pipeline segment covers information on collaborations, acquisition and merger, licensing, patent details, and other information for emerging Bacterial vaginosis therapies.
Bacterial vaginosis Market Forecast Report Insights
- Patient-based Bacterial vaginosis Market Forecasting
- Bacterial vaginosis Therapeutic Approaches
- Bacterial vaginosis Pipeline Analysis
- Bacterial vaginosis Market Size
- Bacterial vaginosis Market Trends
- Bacterial vaginosis Drug Market Opportunities
- Impact of Upcoming Bacterial vaginosis Therapies
Bacterial vaginosis Market Forecast Report Key Strengths
- 11 Years Bacterial vaginosis Market Forecast
- The 7MM Coverage
- Bacterial vaginosis Epidemiology Segmentation
- Key Cross Competition
- Highly Analyzed Bacterial vaginosis Drug Market
- Bacterial Vaginosis Drugs Uptake
Bacterial vaginosis Treatment Market Report Assessment
- Current Bacterial vaginosis Treatment Market Practices
- Current Bacterial Vaginosis Unmet Needs
- Bacterial vaginosis Pipeline Product Profiles
- Bacterial vaginosis Drug Market Attractiveness
- Bacterial vaginosis Market Drivers
- Bacterial vaginosis Market Barriers
Key Questions Answered In The Bacterial vaginosis Market Report:
- How common is bacterial vaginosis?
- What are the key findings of Bacterial vaginosis epidemiology across the 7MM, and which country will have the highest number of patients during the study period (2020–2034)?
- What are the currently available Bacterial Vaginosis treatment?
- What is the disease risk, burden, and bacterial vaginosis unmet needs?
- At what CAGR is the Bacterial vaginosis drug market and its epidemiology expected to grow in the 7MM during the forecast period (2024–2034)?
- How would the unmet needs impact the Bacterial vaginosis market dynamics and subsequently influence the analysis of the related trends?
- What would be the forecasted patient pool of bacterial vaginosis in the 7MM covering the United States, EU4 (Germany, France, Italy, and Spain), the UK, and Japan?
- Among EU4 and the UK, which country will have the highest number of patients during the forecast period (2024–2034)?
- How many companies are currently developing therapies for the treatment of bacterial vaginosis?
Access Exclusive Data Now! Click here to Read More about the Related Articles @ Latest DelveInsight Blog