Bardet-Biedl Syndrome Market
- Bardet-Biedl syndrome (BBS) is a rare autosomal recessive ciliopathy. The disease mapped to at least twenty different genes (BBS1-BBS20), follow oligogenic inheritance pattern.
- Bardet-Biedl syndrome (BBS) is also known by several other names, including: Laurence–Moon–Biedl–Bardet syndrome, Sensenbrenner syndrome, Jeune syndrome.
- Adult Bardet-Biedl syndrome patients often show clinical overlapping with Senior-Løken and Alström Syndromes.
- In 2022, the FDA granted approval for IMCIVREE (setmelanotide) to treat obesity and manage hunger in both adult and pediatric patients aged 6 and above diagnosed with Bardet-Biedl Syndrome (BBS) or Alström syndrome.
- IMCIVREE stands out as the first drug to gain approval for the treatment of Bardet-Biedl Syndrome (BBS) and currently there are no emerging drugs available.
- The market is anticipated to witness a substantial positive shift owing to better uptake of existing drugs and raised awareness.
- The United States accounts for the largest market size of Bardet-Biedl syndrome (BBS), in comparison to EU4 (Germany, Spain, Italy, France), the United Kingdom, and Japan.
DelveInsight's “Bardet-Biedl syndrome (BBS) – Market Insights, Epidemiology and Market Forecast – 2036” report delivers an in-depth understanding of Bardet-Biedl syndrome (BBS), historical and forecasted epidemiology as well as the Bardet-Biedl syndrome (BBS) market trends in the United States, EU4 (Germany, Spain, Italy, and France) and the United Kingdom, and Japan.
Bardet-Biedl syndrome (BBS) market report provides real-world prescription pattern analysis, emerging drugs, market share of individual therapies, and historical and forecasted 7MM Bardet-Biedl syndrome (BBS) market size from 2022 to 2036. The report also covers current Bardet-Biedl syndrome (BBS) treatment practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s underlying potential.
Geography Covered
- The United States
- EU4 (Germany, France, Italy, and Spain) and the United Kingdom
- Japan
|
Study Period |
2022 to 2036 |
|
Forecast Period |
2026-2036 |
|
Geographies Covered |
|
|
Bardet-Biedl syndrome (BBS) Epidemiology |
Segmented by:
|
|
Bardet-Biedl syndrome (BBS) key companies |
|
|
Bardet-Biedl syndrome (BBS) key therapies/drug |
|
Bardet-Biedl syndrome (BBS) Understanding and Treatment Algorithm
Bardet-Biedl syndrome (BBS) Overview, Country-Specific Treatment Guidelines and Diagnosis
Bardet-Biedl syndrome (BBS) is a rare genetic disorder with diverse symptoms affecting multiple body systems. It often includes progressive vision loss leading to legal blindness, early-onset obesity with associated complications like diabetes and high blood pressure, polydactyly (extra fingers or toes), intellectual disability or learning difficulties, and genital abnormalities such as hypogonadism in males and urinary tract malformations in females. Kidney problems are common and can be serious. Other possible features include speech disorders, poor coordination, diabetes insipidus, and heart or liver issues. BBS is typically inherited in an autosomal recessive pattern.
The diagnosis of BBS is based on a combination of clinical features and genetic testing. The clinical diagnosis is supported by the presence of at least four primary features or three primary features and two secondary features. The primary features include: Vision loss, Obesity, Polydactyly, Intellectual disability and learning problems. The secondary features include: Genital abnormalities and Kidney problems.
Genetic testing is also an essential part of the diagnostic process. The diagnostic yield of finding a known causative mutation in a patient diagnosed with BBS is approximately 80%. Genetic testing typically involves a multigene panel or exome sequencing approach.
Further details related to country-based variations in diagnosis are provided in the report
Bardet-Biedl syndrome (BBS) Treatment
For individuals with Bardet-Biedl Syndrome (BBS), a multidisciplinary approach is crucial to address specific symptoms. Early intervention is key to maximizing potential. Counseling during puberty and hormone supplements under endocrinologist guidance may be necessary. Surgical correction can be considered for physical abnormalities like extra digits, genitourinary issues, and heart defects. Careful consideration is needed for anesthesia due to airway anomalies. Obesity management is vital, with diet, exercise, and potential bariatric surgery options. Eye problems require ongoing ophthalmologic care, including correction of refractive errors and regular examinations. Collaboration with schools for special services may aid learning due to visual impairment.
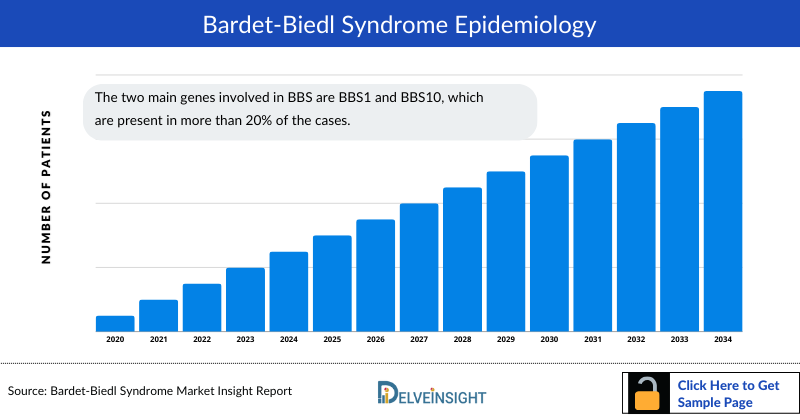
Bardet-Biedl syndrome (BBS) Epidemiology
The Bardet-Biedl syndrome (BBS) epidemiology chapter in the report provides historical as well as forecasted epidemiology in the 7MM covering the United States, EU4 countries (Germany, France, Italy, and Spain), the United Kingdom, and Japan from 2026 to 2036. The Bardet-Biedl syndrome (BBS) epidemiology is segmented with detailed insights into diagnosed incidence cases, mutation-specific cases, and clinical manifestation-specific cases of Bardet-Biedl syndrome (BBS).
- The two main genes involved in BBS are BBS1 and BBS10, which are present in more than 20% of the cases.
- Bardet-Biedl syndrome (BBS) affects males and females in equal numbers.
- Retinal dystrophy is the most penetrant feature, affecting up to 100% of individuals followed by obesity, in 72–92% of cases.
Bardet-Biedl syndrome (BBS) Drug Chapter
The drug chapter segment of the Bardet-Biedl syndrome (BBS) report encloses a detailed analysis of Bardet-Biedl syndrome (BBS) marketed drugs and late-stage (Phase III and Phase II) pipeline drugs. It also deep dives into the Bardet-Biedl syndrome (BBS) pivotal clinical trial details, recent and expected market approvals, patent details, the latest news, and recent deals and collaborations.
Marketed Drugs
IMCIVREE: Rhythm Pharmaceuticals
IMCIVREE (Setmelanotide) is a melanocortin 4 (MC4) receptor agonist indicated for chronic weight management in adult and pediatric patients 6 years of age and older with monogenic or syndromic obesity due to: Pro-opiomelanocortin (POMC), proprotein convertase subtilisin/kexin type 1 (PCSK1), or leptin receptor (LEPR) deficiency as determined by an FDA-approved test demonstrating variants in POMC, PCSK1, or LEPR genes that are interpreted as pathogenic, likely pathogenic, or of uncertain significance (VUS) and in Bardet-Biedl syndrome (BBS).
Setmelanotide is an MC4 receptor agonist with 20-fold less activity at the melanocortin 3 (MC3) and melanocortin 1 (MC1) receptors. MC4 receptors in the brain are involved in regulation of hunger, satiety, and energy expenditure. Based on nonclinical evidence, setmelanotide may re-establish MC4 receptor pathway activity to reduce food intake and promote weight loss through decreased caloric intake and increased energy expenditure in patients with obesity due to POMC, PCSK1, or LEPR deficiency, or BBS associated with insufficient activation of the MC4 receptor. The MC1 receptor is expressed on melanocytes, and activation of this receptor leads to accumulation of melanin and increased skin pigmentation independently of ultraviolet light.
Note: Detailed current therapies assessment will be provided in the full report of Bardet-Biedl syndrome (BBS)
Emerging Drugs
AXV-101: Axovia Therapeutics
AXV-101 is an investigational gene therapy being developed by Axovia Therapeutics to treat BBS — a rare genetic condition that causes progressive vision loss and other symptoms. AXV-101 has received both US FDA Orphan Drug Designation (ODD) and Rare Pediatric Disease Designation (RPDD).
Currently, the AXV-101 is in early Phase I in CT.gov (NCT07269665) and UK clinical trial registry (ISRCTN96250868).
- In January 2026, Axovia Therapeutics reported that it has received an additional USD 1.1 million grant from a Race Against Blindness. This contribution completes the remaining funding requirement and allows the company to move forward with initiating the AXIS (AXV-101) clinical trial in the coming months for children living with BBS type 1.
- In May 2025, Axovia Therapeutics presented new preclinical data for AXV-101, its investigational therapy for the treatment of blindness associated with BBS mutations, at the American Society of Gene & Cell Therapy (ASGCT) annual meeting.
Bardet-Biedl syndrome (BBS) Market Outlook
At present, there are no active trials investigating potential key emerging drugs in the patients with BBS. Symptomatic management is the mainstay approach followed by the physicians.
- The only approved drug is IMCIVREE and currently there are no emerging drugs.
- The unmet needs highlight the importance of continued research, education, and support for individuals with Bardet-Biedl syndrome and their caregivers.
- Bardet–Biedl syndrome is currently treated symptomatically focusing in particular on aggressive management of diabetes, hypertension, and metabolic syndrome to minimize the secondary impact that these conditions have on vulnerable organ systems already affected by BBS, in particular the eyes and kidneys.
KOL Views
To keep up with the real-world scenario in current and emerging market trends, we take opinions from Key Industry leaders working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts were contacted for insights on the evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake along with challenges related to accessibility.
DelveInsight’s analysts connected with 10+ KOLs to gather insights; however, interviews were conducted with 5+ KOLs in the 7MM. Their opinion helps understand and validate current and emerging treatment patterns of Bardet-Biedl syndrome (BBS). This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT analysis and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of gaps in disease diagnosis, patient awareness, physician acceptability, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided.
Conjoint Analysis analyzes multiple approved and emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Market Access and Reimbursement
The report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of currently used therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Scope of the Report
- The report covers a segment of key events, an executive summary, descriptive overview of Bardet-Biedl syndrome (BBS), explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight has been provided into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, and disease progression along with country specific treatment guidelines.
- Additionally, an all-inclusive account of both the current and emerging therapies, along with the elaborative profiles of late-stage and prominent therapies, will have an impact on the current treatment landscape.
- A detailed review of the Bardet-Biedl syndrome (BBS) market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help in shaping and driving the 7MM Bardet-Biedl syndrome (BBS) market.
Bardet-Biedl syndrome (BBS) Report Insights
- Patient Population
- Therapeutic Approaches
- Bardet-Biedl syndrome (BBS) Pipeline Analysis
- Bardet-Biedl syndrome (BBS) Market Size and Trends
- Existing and future Market Opportunity
Bardet-Biedl syndrome (BBS) Report Key Strengths
- Eleven Years Forecast
- 7MM Coverage
- Bardet-Biedl syndrome (BBS) Epidemiology Segmentation
- Inclusion of Country specific treatment guidelines
- KOL’s feedback on approved and emerging therapies
- Key Cross Competition
- Drugs Uptake and Key Market Forecast Assumptions
Bardet-Biedl syndrome (BBS) Report Assessment
- Current Treatment Practices
- Unmet Needs
- Market Attractiveness
- Qualitative Analysis (SWOT)
FAQs
- What is the growth rate of the 7MM Bardet-Biedl syndrome (BBS) treatment market?
- What was the Bardet-Biedl syndrome (BBS) total market size, the market size by therapies, market share (%) distribution in 2022, and what would it look like in 2036? What are the contributing factors/key catalysts for this growth?
- Is there any unexplored patient setting that can open the window for growth in the future?
- What are the pricing variations among different geographies for approved and off-label therapies?
- How would the market drivers, barriers, and future opportunities affect the market dynamics and subsequent analysis of the associated trends? Although multiple expert guidelines recommend testing for targetable mutations before therapy initiation, why do barriers to testing remain high?
- What are the current and emerging options for the treatment of Bardet-Biedl syndrome (BBS)?
- How many companies are developing therapies for the treatment of Bardet-Biedl syndrome (BBS)?
- What are the recent novel therapies, targets, mechanisms of action, and technologies developed to overcome the limitations of existing therapies?
- Patient/physician acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the country-specific accessibility issues of expensive, recently approved therapies?
Reasons to buy
- The report will help in developing business strategies by understanding the latest trends and changing treatment dynamics driving the Bardet-Biedl syndrome (BBS) Market.
- Insights on patient burden/disease prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years
- Understand the existing market opportunities in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identifying strong upcoming players in the market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the conjoint analysis section to provide visibility around leading classes.
- Highlights of access and reimbursement policies of approved therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing market so that the upcoming players can strengthen their development and launch strategy.