Basal Cell Carcinoma Market
- The Basal Cell Carcinoma Market Size is anticipated to grow with a significant CAGR during the study period (2020-2034).
- Basal Cell Carcinoma is the most common type of skin cancer, accounting for approximately 80% of all non-melanoma skin cancers in the United States. It is a locally invasive, keratinocyte cancer that typically occurs on sun-damaged skin, particularly on the head, neck, and trunk.
- According to Roky et al. (2025), in Europe, the Basal Cell Carcinoma incidence rate is 129.3in males and90.8infemales per 100,000 persons per year, with an increasing incidence rate of 5.5%per year and about700,00 new cases of BCC are diagnosed annually in Europe.
- Genetic syndromes such as basal cell naevus syndrome (Gorlin syndrome), Bazex-Dupré-Christol syndrome, Rombo syndrome, Oley syndrome, and xeroderma pigmentosum are associated with an increased risk of Basal Cell Carcinoma.
- PD1 inhibitors, Hedgehog inhibitors, Smoothened (SMO) inhibitors are used in the treatment of Basal cell carcinoma.
- The market is anticipated to witness a substantial positive shift owing to better uptake of existing drugs, the expected market launch of therapies, and raised awareness.
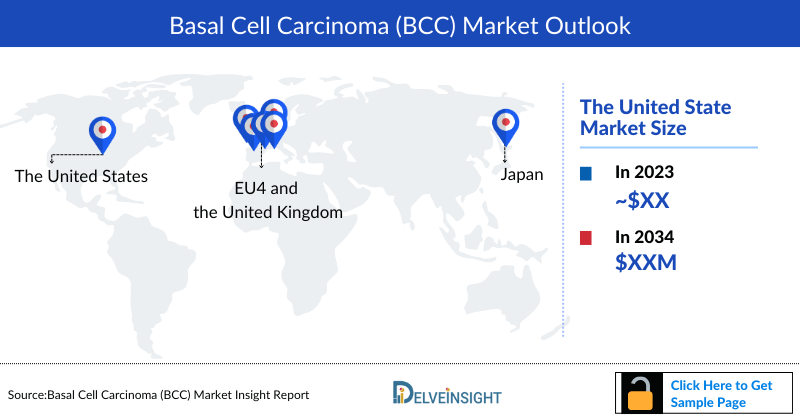
- The United States accounts for the largest market size of Basal Cell Carcinoma, in comparison to EU4 (Germany, Spain, Italy, France), the United Kingdom, and Japan.
- Basal Cell Carcinoma companies are advancing research through targeted therapies and novel drug development to address this common skin cancer. Notable companies include MedC Biopharma Corporation, AiViva BioPharma, MediWound, Kintara Therapeutics, IO Biotech, Sirnaomics, Aresus Pharma, Epitome Pharmaceuticals, Transgene, and others.
- In February 2025, Verrica Pharmaceuticals reported animpressive 97% calculated ORR for VP-315 in treating patients with BCC, the most common cancer type globally. Verrica is preparing for FDA discussions in H1 2025 to outline the path toward a Phase III trial.
- In February 2025, Medicus Pharma CEO Dr. Raza Bokhari announced significant progress in the company's Phase 2 clinical study, which is being conducted at nine clinical sites across the United States. The study has now randomized more than 50% of its targeted 60 patients.
- In January 2025, Stamford Pharmaceuticals announced positive results for its ASN-002-003 Phase II a trial evaluatingSP-002 in combination with vismodegib in subjects presenting with multiple Basal Cell Carcinoma.
- In November 2024, US-based company Biofrontera announced the topline results from a Phase III trial assessing the effectiveness of its drug-device therapy, Ameluz gel combined with photodynamic therapy (PDT) using the BF-RhodoLED lamp, for the treatment of superficial basal cell carcinoma.
- In April 2024, Stamford Pharmaceuticals announced the initiation of Phase II study combining SP-002 with ERIVEDGE in locally advanced basal cell carcinoma patients.
- In March 2024, Medicus Pharma received US FDA comments on Phase II clinical protocol for non-invasive treatment of basal cell carcinoma using patented dissolvable microneedle patch to deliver chemotherapeutic agent to eradicate tumors cells.
- In December 2023, AiViva Biopharma, completed their initial trial administering AIV001 (axitinib) to patients with Basal Cell Carcinoma tumors.
- ERIVEDGE, ODOMZO, LIBTAYO, EFUDEX, and ZYCLARA are some of the approved therapies for Basal Cell Carcinoma.
- Basal Cell Carcinoma pipeline possesses potential drugs such as CX-4945, SP-002, AIV001, and patidegib.
Request for Sample Page @ Basal Cell Carcinoma Market Report
DelveInsight's “Basal Cell Carcinoma Market Insights, Epidemiology and Market Forecast – 2034” report delivers an in-depth understanding of Basal Cell Carcinoma, historical and forecasted epidemiology as well as the Basal Cell Carcinoma therapeutics market trends in the United States, EU4 (Germany, Spain, Italy, and France) and the United Kingdom, and Japan.
Basal Cell Carcinoma market report provides real-world prescription pattern analysis, emerging drugs, market share of individual therapies, and historical and forecasted 7MM Basal Cell Carcinoma market size from 2020 to 2034. The report also covers current Basal Cell Carcinoma treatment practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s underlying potential.
|
Study Period |
2020 to 2034 |
|
Forecast Period |
2024-2034 |
|
Geographies Covered |
The US, EU4 (Germany, France, Italy, and Spain) and UK, Japan |
|
Basal Cell Carcinoma Market |
|
|
Basal Cell Carcinomas Market Size | |
|
Basal Cell Carcinoma Companies |
MedC Biopharma Corporation, AiViva BioPharma, MediWound, Kintara Therapeutics, IO Biotech, Sirnaomics, Aresus Pharma, Epitome Pharmaceuticals, Transgene, Senhwa Biosciences, Palvella Therapeutics, Suzhou Kintor Pharmaceuticals, Leaf Vertical, and others. |
|
Basal Cell Carcinoma Epidemiology Segmentation |
|
Key Factors Driving the Growth of the Basal Cell Carcinoma Market
Rising Incidence and Aging Population
The global incidence of basal cell carcinoma is increasing due to prolonged exposure to ultraviolet (UV) radiation, genetic predispositions, and an aging population that is more susceptible to skin cancers. As people live longer and spend more time outdoors, the number of diagnosed cases continues to rise, fueling demand for effective treatments.
Technological Advancements and Treatment Innovations
Recent years have seen significant progress in BCC treatment modalities, including targeted therapies (such as hedgehog pathway inhibitors like vismodegib and sonidegib), immunotherapies, and personalized medicine approaches. Minimally invasive options, such as intralesional injections and topical treatments, are gaining popularity due to their reduced side effects and improved patient outcomes. The integration of artificial intelligence (AI) and digital health solutions is also enhancing early diagnosis and treatment planning.
Launch of Emerging BCC Drugs
The expected launch of therapies such as Remetinostat (Medvir), STP705 (Sirnaomics), SP-002 (Stamford Pharmaceuticals), LTX-315/VP-315 (Lytix Biopharma/Verrica Pharmaceuticals), and others will change the dynamics of the BCC market.
Basal Cell Carcinoma Treatment Market
Basal Cell Carcinoma Overview, Country-Specific Treatment Guidelines and Diagnosis
Basal Cell Carcinoma primarily stems from UV radiation exposure, leading to mutations in the PTCH tumor suppressor gene. Other risk factors include age, gender, previous skin cancers, sun damage, fair complexion, inherited syndromes like Gorlin syndrome, and factors like ionizing radiation exposure, arsenic exposure, immune suppression, and certain medication use.
The diagnosis of Basal Cell Carcinoma typically involves a combination of clinical evaluation and histopathological confirmation. Clinically, Basal Cell Carcinomas often present as slow-growing plaques or nodules with varying sizes, colors, and locations, while histopathological examination is necessary for definitive diagnosis and to determine the histological subtype.
Further details related to country-based variations in diagnosis are provided in the report
Basal Cell Carcinoma Treatment
Treatment options for Basal Cell Carcinoma include surgical excision, cryotherapy, radiation therapy, and topical treatments such as imiquimod and 5-fluorouracil. Micrographic surgery is particularly effective for high-risk Basal Cell Carcinomas and those with unclear borders or aggressive growth patterns. Laser therapy and electric cauterization and curettage are also used for smaller tumors. Regular follow-up care is crucial to monitor for potential recurrences and new skin cancers. Basal Cell Carcinoma clinical trials are essential for evaluating new therapies, improving treatment outcomes, and advancing our understanding of disease mechanisms to develop more effective, targeted, and safer interventions for patients.
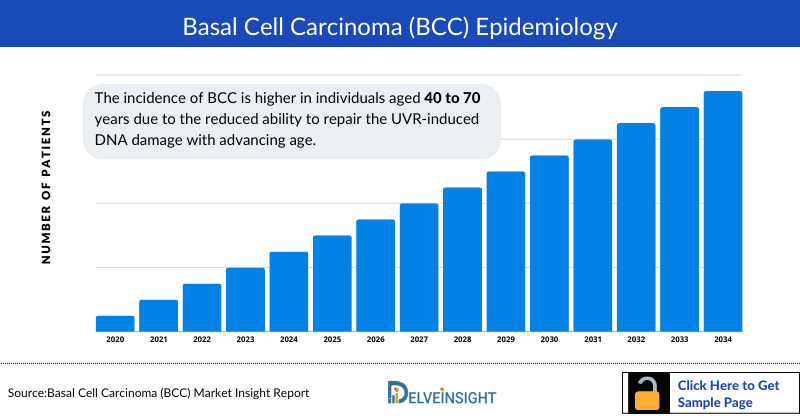
Basal Cell Carcinoma Epidemiology
The Basal Cell Carcinoma epidemiology chapter in the report provides historical as well as forecasted epidemiology in the 7MM covering the United States, EU4 countries (Germany, France, Italy, and Spain), the United Kingdom, and Japan from 2024 to 2034. The Basal Cell Carcinoma epidemiology is segmented with detailed insights into incidence cases, age-specific cases, gender-specific cases, mutation-specific cases, site-specific cases, and histological type-specific cases of Basal Cell Carcinoma.
- The incidence of Basal Cell Carcinoma is higher in individuals aged 40 to 70 years due to the reduced ability to repair the UVR-induced DNA damage with advancing age.
- Women under 40 have a higher incidence of Basal Cell Carcinoma, while in older age, the disease is predominantly observed in men.
- In approximately 80% of the cases Basal Cell Carcinoma is located on the face and neck.
- Approximately 60% of both familial and sporadic cases of Basal Cell Carcinomas exhibit genetic alterations within the microsatellite D6S251 located in the 6q14 region.
- American Cancer Society (2023), reports skin cancer as being the most common cancer in the US, with Basal Cell Carcinoma constituting the majority of cases. According to the estimate that about 5.4 million basal and squamous cell skin cancers (SCC) are diagnosed each year in about 3.3 million persons in the US, with about 80% of those being Basal Cell Carcinoma.
- As per Skin Cancer foundation (2023), BCC is the most common form of skin cancer. An estimated 3.6million cases of Basal Cell Carcinoma are diagnosed in the US each year.
- As noted by Schmults (2023), basal cell carcinomas rarely metastasize, with a rate of less than0.1%, and are generally associated with a positive prognosis.
- As per Desai et al. (2022), in Europe, the incidence has risen at a rate of 5%per year over the lastdecade compared to approximately 2% in the US. Nodular Basal Cell Carcinoma (nBCC) subtype accounts for60–80%ofall Basal Cell Carcinoma.
According to Cameron et al. (2019), approximately 80% of skin carcinomas cases have been attributedto BCC.
Basal Cell Carcinoma Recent Developments
- In February 2025, Verrica Pharmaceuticals reported animpressive 97% calculated ORR for VP-315 in treating patients with BCC, the most common cancer type globally. Verrica is preparing for FDA discussions in H1 2025 to outline the path toward a Phase III trial.
- In February 2025, Medicus Pharma CEO Dr. Raza Bokhari announced significant progress in the company's Phase 2 clinical study, which is being conducted at nine clinical sites across the United States. The study has now randomized more than 50% of its targeted 60 patients.
- In January 2025, Stamford Pharmaceuticals announced positive results for its ASN-002-003 Phase II a trial evaluatingSP-002 in combination with vismodegib in subjects presenting with multiple Basal Cell Carcinoma.
- In November 2024, US-based company Biofrontera announced the topline results from a Phase III trial assessing the effectiveness of its drug-device therapy, Ameluz gel combined with photodynamic therapy (PDT) using the BF-RhodoLED lamp, for the treatment of superficial basal cell carcinoma (sBasal Cell Carcinoma).
Basal Cell Carcinoma Drug Chapter
The drug chapter segment of the Basal Cell Carcinoma report encloses a detailed analysis of Basal Cell Carcinoma marketed drugs and late-stage (Phase III and Phase II) Basal Cell Carcinoma pipeline drugs. It also deep dives into the Basal Cell Carcinoma pivotal clinical trial details, recent and expected market approvals, patent details, the latest news, and recent deals and collaborations. The Basal Cell Carcinoma drugs market is expanding steadily due to rising incidences, increased awareness, and ongoing advancements in targeted therapies and immuno-oncology treatment options for skin cancer management.
Basal Cell Carcinoma Marketed Drugs
LIBTAYO: Regeneron Pharmaceuticals
LIBTAYO (cemiplimab-rwlc) is a programmed death receptor-1 (PD-1) blocking antibody indicated for Cutaneous Squamous Cell Carcinoma (CSCC), Basal Cell Carcinoma and Non-Small Cell Lung Cancer (NSCLC).
Cemiplimab-rwlc, a recombinant human IgG4 monoclonal antibody, disrupts the interaction between PD-1 receptor on T cells and its ligands, PD-L1 and PD-L2. By doing so, it prevents the inhibition of T-cell proliferation and cytokine production. This release of PD-1 pathway-mediated inhibition boosts the anti-tumor immune response, leading to decreased tumor growth.
ERIVEDGE: Genentech
ERIVEDGE (vismodegib) is a hedgehog pathway inhibitor indicated for the treatment of adults with metastatic basal cell carcinoma, or with locally advanced basal cell carcinoma that has recurred following surgery or who are not candidates for surgery and who not candidates for radiation are. The recommended dosage is 150 mg orally once daily. Vismodegib is an inhibitor of the Hedgehog pathway. Vismodegib binds to and inhibits Smoothened, a transmembrane protein involved in Hedgehog signal transduction.
Note: Detailed current therapies assessment will be provided in the full report of Basal Cell Carcinoma...
Emerging Basal Cell Carcinoma Drugs
LTX-315/VP-315: Lytix Biopharma/Verrica Pharmaceuticals
LTX-315 is an oncolytic molecule designed for direct tumor injection. It works by killing cancer cells and stimulating the immune system to boost the antitumor response. Preclinical studies show it can inhibittumor growth, induce regression, and provide lasting immune protection. A key benefit of LTX-315 is itsability to promote T-cell infiltration into tumors. This process helps target and destroy cancer cells.
- In February 2025, Verrica Pharmaceuticals reported an impressive 97% calculated ORR in treatingpatients with BCC, the most common cancer type globally. Verrica is preparing for FDA discussions inH1 2025 to outline the path toward a Phase III trial.
SP-002: Stamford Pharmaceuticals
SP-002 is a biologic therapeutic based on an adenovirus (a type of cold-virus) that has been engineered to produce the immunostimulatory anti-cancer protein, Interferon-g. Stamford has an exclusive worldwide license to develop SP-002 (formerly TG1042) from the French biopharmaceutical company Transgene.
- As per Stamford Pharmaceuticals recent pipeline updates, the company is planning to commence Phase III of SP-002 for BCC in 2H 2025/1H 2026.
- In January 2025, Stamford Pharmaceuticals announced positive results for its ASN-002-003 Phase IIa trial evaluating SP-002 in combination with isomeric in subjects presenting with multiple BCCs.
STP705: Sirnaomics
STP705, a product candidate developed by Sirnaomics, is a dual TGF-ß1/COX-2 inhibitor. TGF-ß1 andCOX-2 are well-validated gatekeeper targets for oncology and fibrosis disease drug development. The drug is intended to cause downregulation of the expression of these genes to inhibit tumor growth, providing an alternative to surgical excision or other invasive treatment of BCC.
- The latest results from the Phase II clinical study of STP705 for BCC treatment, showing an incredible efficacy without any drug-related adverse effect and serious adverse effects, further validated the broad potential of this drug candidate for treatment of non melanoma skin cancers and beyond.
- In August 2022, the company announced that the cohort receiving the 180 μg dose level in the PhaseII clinical trial of STP705 for the treatment of cutaneous BCC achieved a 100% complete response.The product is currently in the pipeline, but there have been no further updates regarding its development.
Patidegib (SGT-610) : Sol-Gel Technologies
Patidegib is an investigational small molecule that inhibits hedgehog signaling. It is a topical treatment designed to mitigate the tumor burden in patients with Gorlin Syndrome and Basal Cell Carcinomas (Basal Cell Carcinomas), and other potential indications. Topical patidegib is a first-in-class topical gel formulation of a proprietary hedgehog inhibitor exclusively licensed from Infinity Pharmaceuticals. Patidegib is currently evaluated in Phase III clinical trials for the treatment of Basal Cell Carcinomas.
In January 2023, Sol-Gel Technologies acquired topically-applied patidegib from PellePharm for treating Gorlin syndrome, a rare condition lacking FDA or EMA-approved therapies. This adds investigational compound SGT-610 to Sol-Gel's pipeline, potentially the first treatment for Gorlin syndrome. Gorlin syndrome, or nevoid Basal Cell Carcinoma syndrome, leads to multiple Basal Cell Carcinomas. Current treatment involves painful surgical excision, but preventing new Basal Cell Carcinoma formation is essential due to the impracticality of repeated surgeries. SGT-610 aims to address this without the systemic adverse effects of oral Basal Cell Carcinoma therapies.
AIV001: AiViva BioPharma
AIV001 is a novel formulation of a multi-kinase inhibitor combined with AiViva's proprietary delivery technology, designed for prolonged drug release via intradermal treatment. AIV001 targets multiple pathways to reduce fibroplasia in overlapping phases of wound healing and scarring; targets VEGFR to limit the inflammation and fibrosis associated with rosacea; and inhibits or reduces neovascularization and cell proliferation associated with certain cancers. AIV001 is in Phase I/II clinical studies for the treatment of patients with Basal Cell Carcinoma.
Discover more on the Photodynamic Therapies @ Photodynamic Therapies in Oncology
Note: Detailed emerging therapies assessment will be provided in the final report...
Basal Cell Carcinoma Market Outlook
Key Basal Cell Carcinoma companies, such as PellePharm, AiViva BioPharma, Sol-Gel Technologies, Stamford Pharmaceuticals, Senhwa Biosciences, and others are evaluating their lead candidates in different stages of clinical development, respectively. They aim to investigate their products for the treatment of Basal Cell Carcinoma.
- Surgical excision is the most common treatment for Basal Cell Carcinoma, but other options like topical medications, radiation therapy, and hedgehog inhibitors are also gaining popularity.
- The market's expansion is hindered by substantial expenses linked to treating Basal Cell Carcinoma and the delayed diagnosis attributed to their late clinical presentation.
- The market is driven by the rising prevalence of skin malignancies, increasing demand for effective treatments, and governmental initiatives aimed at controlling skin cancer.
Basal Cell Carcinoma Drugs Uptake
This section focuses on the uptake rate of potential drugs expected to be launched in the market during 2024–2034, which depends on the competitive landscape, safety, and efficacy data along with order of entry. It is important to understand that the key players evaluating their novel therapies in the pivotal and confirmatory trials should remain vigilant when selecting appropriate comparators to stand the greatest chance of a positive opinion from regulatory bodies, leading to approval, smooth launch, and rapid uptake.
Further detailed analysis of emerging therapies drug uptake in the report...
Basal Cell Carcinoma Activities
The Basal Cell Carcinoma pipeline report provides insights into Basal Cell Carcinoma clinical trials within Phase III and Phase II stages. It also analyzes key players involved in developing targeted therapeutics.
Basal Cell Carcinoma Pipeline Development Activities
The Basal Cell Carcinoma clinical trials analysis report covers information on collaborations, acquisitions and mergers, licensing, and patent details for Basal Cell Carcinoma emerging therapies.
KOL Views
To keep up with the real-world scenario in current and emerging market trends, we take opinions from Key Industry leaders working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts were contacted for insights on the evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake along with challenges related to accessibility.
DelveInsight’s analysts connected with 40+ KOLs to gather insights; however, interviews were conducted with 25+ KOLs in the 7MM. Their opinion helps understand and validate current and emerging treatment patterns of Basal Cell Carcinoma. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT analysis and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of gaps in disease diagnosis, patient awareness, physician acceptability, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided.
Conjoint Analysis analyzes multiple approved and emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Market Access and Reimbursement
The report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of currently used therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Scope of the Basal Cell Carcinoma Market Report
- The report covers a segment of key events, an executive summary, descriptive overview of Basal Cell Carcinoma, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight has been provided into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, and disease progression along with country specific treatment guidelines.
- Additionally, an all-inclusive account of both the current and emerging therapies, along with the elaborative profiles of late-stage and prominent therapies, will have an impact on the current treatment landscape.
- A detailed review of the Basal Cell Carcinoma market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help in shaping and driving the 7MM Basal Cell Carcinoma market.
Basal Cell Carcinoma Market Report Insights
- Basal Cell Carcinoma Patient Population
- Basal Cell Carcinoma Therapeutic Market
- Basal Cell Carcinoma Pipeline Analysis
- Basal Cell Carcinoma Market Size and Trends
- Existing and future Market Opportunity
Basal Cell Carcinoma Market Report Key Strengths
- Eleven Years Forecast
- 7MM Coverage
- Basal Cell Carcinoma Epidemiology Segmentation
- Inclusion of Country specific treatment guidelines
- KOL’s feedback on approved and emerging therapies
- Key Cross Competition
- Conjoint analysis
- Basal Cell Carcinoma Drugs Uptake
- Key Basal Cell Carcinoma Market Forecast Assumptions
Basal Cell Carcinoma Market Report Assessment
- Current Basal Cell Carcinoma Treatment Practices
- Basal Cell Carcinoma Unmet Needs
- Basal Cell Carcinoma Pipeline Product Profiles
- Basal Cell Carcinoma Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
- Basal Cell Carcinoma Market Drivers
- Basal Cell Carcinoma Market Barriers
FAQs
- What is the growth rate of the 7MM Basal Cell Carcinoma treatment market?
- What was the Basal Cell Carcinoma total market size, the market size by therapies, market share (%) distribution in 2020, and what would it look like in 2034? What are the contributing factors/key catalysts for this growth?
- Is there any unexplored patient setting that can open the window for growth in the future?
- What are the pricing variations among different geographies for approved and off-label therapies?
- How would the market drivers, barriers, and future opportunities affect the market dynamics and subsequent analysis of the associated trends? Although multiple expert guidelines recommend testing for targetable mutations before therapy initiation, why do barriers to testing remain high?
- What are the current and emerging options for the treatment of Basal Cell Carcinoma?
- How many Basal Cell Carcinoma companies are developing therapies for the treatment of Basal Cell Carcinoma?
- What are the recent novel therapies, targets, mechanisms of action, and technologies developed to overcome the limitations of existing therapies?
- Patient/physician acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the country-specific accessibility issues of expensive, recently approved therapies?
Reasons to buy Basal Cell Carcinoma Market Forecast Report
- The report will help in developing business strategies by understanding the latest trends and changing treatment dynamics driving the Basal Cell Carcinoma Market.
- Insights on patient burden/disease prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years
- Understand the existing market opportunities in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identifying strong upcoming players in the market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the conjoint analysis section to provide visibility around leading classes.
- Highlights of access and reimbursement policies of approved therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing market so that the upcoming players can strengthen their development and launch strategy.
-market.png&w=256&q=75)

-pipeline.png&w=256&q=75)
