BCG-unresponsive Non Muscle Invasive Bladder Cancer Market
- BCG-unresponsive non-muscle invasive bladder cancer (BCG-unresponsive NMIBC) refers to cases where patients do not respond to Bacille Calmette-Guérin (BCG) therapy, which is commonly used to prevent or delay tumor recurrence following Non Muscle Invasive Bladder Cancer (NMIBC) resection.
- The market is anticipated to witness a substantial positive shift owing to better uptake of existing drugs, the expected market launch of photodynamic therapy and non-viral gene therapy, and raised awareness.
- The United States accounts for the largest market size of BCG-unresponsive Non Muscle Invasive Bladder Cancer, in comparison to EU4 (Germany, Spain, Italy, France), the United Kingdom, and Japan.
- In April 2024, The FDA approved ANKTIVA (N-803, or nogapendekin alfa inbakicept-pmln) in combination with Bacillus Calmette-Guérin (BCG) for the treatment of patients with BCG-unresponsive, non-muscle invasive bladder cancer (NMIBC) with carcinoma in situ, with or without papillary tumors. ANKTIVA, is first-in-class interleukin-15 agonist immunotherapy for NMIBC.
- In December 2023, Johnson & Johnson announced that the FDA had granted TAR-200 Breakthrough Therapy Designation (BTD) for the potential future treatment of patients with Bacillus Calmette-Guérin (BCG)-unresponsive high-risk non-muscle-invasive bladder cancer (HR-NMIBC), who were ineligible for or elected not to undergo radical cystectomy (surgical removal of the bladder).
- BCG-unresponsive Non Muscle Invasive Bladder Cancer pipeline possesses potential drugs like TAR-200, EG-70, intravesical Photodynamic Therapy and others.
DelveInsight's “BCG-unresponsive Non Muscle Invasive Bladder Cancer (BCG-unresponsive NMIBC) – Market Insights, Epidemiology and Market Forecast – 2034” report delivers an in-depth understanding of BCG-unresponsive Non Muscle Invasive Bladder Cancer , historical and forecasted epidemiology as well as the BCG-unresponsive Non Muscle Invasive Bladder Cancer market trends in the United States, EU4 (Germany, Spain, Italy, and France) and the United Kingdom, and Japan.
BCG-unresponsive Non Muscle Invasive Bladder Cancer market report provides real-world prescription pattern analysis, emerging drugs, market share of individual therapies, and historical and forecasted 7MM BCG-unresponsive Non Muscle Invasive Bladder Cancer market size from 2020 to 2034. The report also covers current BCG-unresponsive Non Muscle Invasive Bladder Cancer treatment practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s underlying potential.
Geography Covered
- The United States
- EU4 (Germany, France, Italy, and Spain) and the United Kingdom
- Japan
|
Study Period |
2020 to 2034 |
|
Forecast Period |
2024-2034 |
|
Geographies Covered |
|
|
BCG-unresponsive Non Muscle Invasive Bladder Cancer Epidemiology |
Segmented by:
|
|
BCG-unresponsive Non Muscle Invasive Bladder Cancer key companies |
|
|
BCG-unresponsive Non Muscle Invasive Bladder Cancer key therapies/drug |
|
|
BCG-unresponsive Non Muscle Invasive Bladder Cancer Analysis |
KOL Views
|
BCG-unresponsive Non Muscle Invasive Bladder Cancer Understanding and Treatment Algorithm
BCG-unresponsive Non Muscle Invasive Bladder Cancer Overview, Country-Specific Treatment Guidelines and Diagnosis
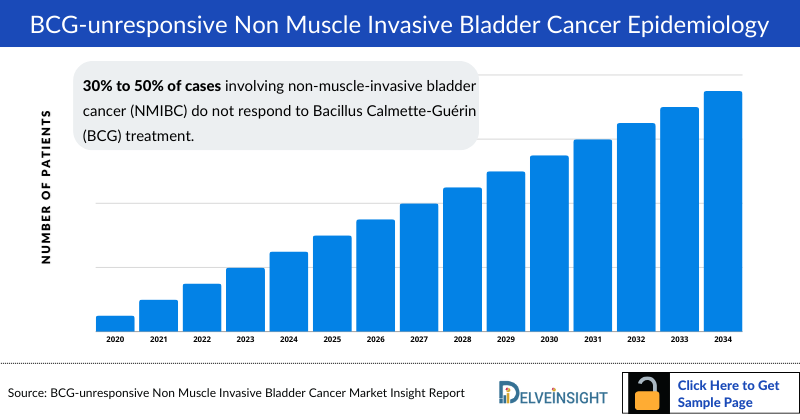
BCG-unresponsive non-muscle invasive bladder cancer (NMIBC) is characterized by the failure of Bacille Calmette-Guérin (BCG) therapy to control the disease. It's defined by the FDA as high-grade T1 disease after initial BCG treatment, recurrent high-grade Ta/T1 disease within six months post-BCG therapy, or persistent/recurrent carcinoma in situ (CIS) within 12 months post-treatment. Incidence rates range from 30% to 50%. Diagnosis involves clinical evaluation, pathology, and imaging, focusing on factors like pathological staging, clinical response to BCG therapy, and imaging findings to assess disease progression and treatment response.
Further details related to country-based variations in diagnosis are provided in the report
BCG-unresponsive Non Muscle Invasive Bladder Cancer Treatment
Current treatment options for high-risk BCG-unresponsive non-muscle invasive bladder cancer include radical cystectomy, intravesical therapy with chemotherapeutic agents like gemcitabine and docetaxel for patients unfit for surgery, immunotherapy with checkpoint inhibitors such as pembrolizumab, and ongoing exploration of device-assisted therapy including new intravesical and systemic agents in combination with conventional treatments.
BCG-unresponsive Non Muscle Invasive Bladder Cancer Epidemiology
The BCG-unresponsive Non Muscle Invasive Bladder Cancer epidemiology chapter in the report provides historical as well as forecasted in the 7MM covering the United States, EU4 countries (Germany, France, Italy, and Spain), the United Kingdom, and Japan from 2024 to 2034. The BCG-unresponsive Non Muscle Invasive Bladder Cancer epidemiology is segmented with detailed insights into prevalence, age-specific, gender-specific, risk factor- specific, line-wise treated case of BCG-unresponsive Non Muscle Invasive Bladder Cancer.
- 30% to 50% of cases involving non-muscle-invasive bladder cancer (NMIBC) do not respond to Bacillus Calmette-Guérin (BCG) treatment.
- The likelihood of BCG-unresponsive NMIBC rises with age, especially among individuals aged 65 and older.
- Men exhibit a higher susceptibility to developing BCG-unresponsive NMIBC compared to women.
BCG-unresponsive Non Muscle Invasive Bladder Cancer Recent Developments
- In April 2025, ImmunityBio announced multiple submissions to the FDA, including a supplemental Biologics License Application (sBLA) for BCG-unresponsive non-muscle invasive bladder cancer (NMIBC) in papillary disease and an Expanded Access Program (EAP) for ANKTIVA® (nogapendekin alfa inbakicept-pmln) for lymphopenia treatment.
BCG-unresponsive Non Muscle Invasive Bladder Cancer Drug Chapter
The drug chapter segment of the BCG-unresponsive Non Muscle Invasive Bladder Cancer report encloses a detailed analysis of BCG-unresponsive Non Muscle Invasive Bladder Cancer marketed drugs and late-stage (Phase III and Phase II) pipeline drugs. It also deep dives into the BCG-unresponsive Non Muscle Invasive Bladder Cancer pivotal clinical trial details, recent and expected market approvals, patent details, the latest news, and recent deals and collaborations.
Marketed Drugs
ANKTIVA: Altor Bioscience
ANKTIVA is an interleukin-15 (IL-15) receptor agonist indicated with Bacillus Calmette-Guérin (BCG) for the treatment of adult patients with BCG unresponsive non-muscle invasive bladder cancer (NMIBC) with carcinoma in situ (CIS) with or without papillary tumors. ANKITVA activates NK, CD8+, and memory T cells without stimulating immunosuppressive Treg cells. It binds to the IL-15 receptor, composed of the common gamma chain (γc), beta chain (βc), and IL-15-specific alpha subunit (IL-15Rα), leading to cell proliferation and activation. In preclinical studies using a rat model of bladder cancer, intravesical administration of ANKITVA (nogapendekin alfa inbakicept-pmln) alone or combined with BCG demonstrated superior anti-tumor activity compared to BCG alone. In April 2024, The FDA approved ANKTIVA.
KEYTRUDA: Merck Sharp & Dohme
In 2020, FDA approved KEYTRUDA (pembrolizumab), a programmed death receptor-1 (PD-1)-blocking antibody, as a single agent for the treatment of patients with Bacillus Calmette-Guerin (BCG)-unresponsive, high-risk, non-muscle invasive bladder cancer (NMIBC) with carcinoma in situ (CIS) with or without papillary tumors who are ineligible for or have elected not to undergo cystectomy. Binding of the PD-1 ligands, PD-L1 and PD-L2, to the PD-1 receptor found on T cells, inhibits T cell proliferation and cytokine production. Upregulation of PD-1 ligands occurs in some tumors and signaling through this pathway can contribute to inhibition of active T-cell immune surveillance of tumors. Pembrolizumab is a monoclonal antibody that binds to the PD-1 receptor and blocks its interaction with PD-L1 and PD-L2, releasing PD-1 pathway-mediated inhibition of the immune response, including the anti-tumor immune response.
Note: Detailed current therapies assessment will be provided in the full report of BCG-unresponsive Non Muscle Invasive Bladder Cancer
Emerging Drugs
TAR-200 (RIS/gemcitabine plus cetrelimab): Johnson & Johnson
TAR-200, a novel investigational targeted releasing system designed to provide sustained local release of gemcitabine into the bladder. TAR-200 is a targeted releasing system designed for controlled release of gemcitabine into the bladder, prolonging local drug exposure. Cetrelimab is an investigational PD-1 monoclonal antibody under study for bladder cancer, prostate cancer, melanoma, and multiple myeloma treatment. The drug is currently in Phase II.
In December 2023, Johnson & Johnson announced that the FDA had granted TAR-200 Breakthrough Therapy Designation (BTD) for the potential future treatment of patients with Bacillus Calmette-Guérin (BCG)-unresponsive high-risk non-muscle-invasive bladder cancer (HR-NMIBC), who were ineligible for or elected not to undergo radical cystectomy (surgical removal of the bladder).
EG-70: enGene
EG-70 (detalimogene voraplasmid) is a novel, non-viral gene therapy being investigated for the treatment of Bacillus Calmette-Guérin (BCG)-unresponsive, high-risk non-muscle invasive bladder cancer (NMIBC) with carcinoma in situ (CIS). EG-70 is engineered to stimulate both the innate and adaptive immune responses within the bladder. Its goal is to induce a coordinated immune reaction within the bladder, targeting tumors for elimination.
EG-70 has demonstrated promising efficacy and safety in patients with BCG-unresponsive, high-risk NMIBC CIS, with high complete response rates and a favorable tolerability profile. Currently being investigated in Phase I/II trials.
Note: Detailed emerging therapies assessment will be provided in the final report.
|
Therapy Name |
Company Name |
ROA |
MOA |
Phases |
Any Special Status |
|
TAR-200 |
Johnson & Johnson |
Intravesical Delivery System |
Prolonged exposure to anti-cancer chemotherapy drug |
II |
Breakthrough Therapy Designation |
|
EG-70 |
enGene |
Intravesical |
Gene therapy |
I/II |
Fast Track Designation |
BCG-unresponsive Non Muscle Invasive Bladder Cancer Market Outlook
Key players, such as Theralase Technologies, enGene, Janssen Research & Development and others are evaluating their lead candidates in different stages of clinical development, respectively. They aim to investigate their products for the treatment of BCG-unresponsive Non Muscle Invasive Bladder Cancer.
- Immune checkpoint inhibitors like pembrolizumab, have shown promising results in clinical trials for patients with BCG-unresponsive NMIBC.
- Several inhibitors are being evaluating for BCG unresponsive NMIBC like IDO1 inhibitor, FGFR inhibitor, PD-L1 inhibitor, PD-1 inhibitor and others.
- The market outlook is positive, with recent FDA approvals and a robust pipeline of novel therapies for BCG-unresponsive NMIBC.
BCG-unresponsive Non Muscle Invasive Bladder Cancer Drugs Uptake
This section focuses on the uptake rate of potential drugs expected to be launched in the market during 2024–2034, which depends on the competitive landscape, safety, and efficacy data along with order of entry. It is important to understand that the key players evaluating their novel therapies in the pivotal and confirmatory trials should remain vigilant when selecting appropriate comparators to stand the greatest chance of a positive opinion from regulatory bodies, leading to approval, smooth launch, and rapid uptake.
Further detailed analysis of emerging therapies drug uptake in the report…
BCG-unresponsive Non Muscle Invasive Bladder Cancer Activities
The report provides insights into different therapeutic candidates in Phase III and Phase II stages. It also analyzes key players involved in developing targeted therapeutics.
Pipeline Development Activities
The report covers information on collaborations, acquisitions and mergers, licensing, and patent details for BCG-unresponsive Non Muscle Invasive Bladder Cancer emerging therapies.
KOL Views
To keep up with the real-world scenario in current and emerging market trends, we take opinions from Key Industry leaders working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts were contacted for insights on the evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake along with challenges related to accessibility.
DelveInsight’s analysts connected with 40+ KOLs to gather insights; however, interviews were conducted with 25+ KOLs in the 7MM. Their opinion helps understand and validate current and emerging treatment patterns of BCG-unresponsive Non Muscle Invasive Bladder Cancer. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT analysis and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of gaps in disease diagnosis, patient awareness, physician acceptability, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided.
Conjoint Analysis analyzes multiple approved and emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Market Access and Reimbursement
The report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of currently used therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Scope of the Report
- The report covers a segment of key events, an executive summary, descriptive overview of BCG-unresponsive Non Muscle Invasive Bladder Cancer, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight has been provided into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, and disease progression along with country specific treatment guidelines.
- Additionally, an all-inclusive account of both the current and emerging therapies, along with the elaborative profiles of late-stage and prominent therapies, will have an impact on the current treatment landscape.
- A detailed review of the BCG-unresponsive Non Muscle Invasive Bladder Cancer market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help in shaping and driving the 7MM BCG-unresponsive Non Muscle Invasive Bladder Cancer market.
BCG-unresponsive Non Muscle Invasive Bladder Cancer Report Insights
- Patient Population
- Therapeutic Approaches
- BCG-unresponsive Non Muscle Invasive Bladder Cancer Pipeline Analysis
- BCG-unresponsive Non Muscle Invasive Bladder Cancer Market Size and Trends
- Existing and future Market Opportunity
BCG-unresponsive Non Muscle Invasive Bladder Cancer Report Key Strengths
- Eleven Years Forecast
- 7MM Coverage
- BCG-unresponsive Non Muscle Invasive Bladder Cancer Epidemiology Segmentation
- Inclusion of Country specific treatment guidelines
- KOL’s feedback on approved and emerging therapies
- Key Cross Competition
- Conjoint analysis
- Drugs Uptake and Key Market Forecast Assumptions
BCG-unresponsive Non Muscle Invasive Bladder Cancer Report Assessment
- Current Treatment Practices
- Unmet Needs
- Pipeline Product Profiles
- Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
FAQs
- What is the growth rate of the 7MM BCG-unresponsive Non Muscle Invasive Bladder Cancer treatment market?
- What was the BCG-unresponsive Non Muscle Invasive Bladder Cancer total market size, the market size by therapies, market share (%) distribution in 2020, and what would it look like in 2034? What are the contributing factors/key catalysts for this growth?
- Is there any unexplored patient setting that can open the window for growth in the future?
- What are the pricing variations among different geographies for approved and off-label therapies?
- How would the market drivers, barriers, and future opportunities affect the market dynamics and subsequent analysis of the associated trends? Although multiple expert guidelines recommend testing for targetable mutations before therapy initiation, why do barriers to testing remain high?
- What are the current and emerging options for the treatment of BCG-unresponsive Non Muscle Invasive Bladder Cancer?
- How many companies are developing therapies for the treatment of BCG-unresponsive Non Muscle Invasive Bladder Cancer?
- What are the recent novel therapies, targets, mechanisms of action, and technologies developed to overcome the limitations of existing therapies?
- Patient/physician acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the country-specific accessibility issues of expensive, recently approved therapies?
Reasons to buy
- The report will help in developing business strategies by understanding the latest trends and changing treatment dynamics driving the BCG-unresponsive Non Muscle Invasive Bladder Cancer Market.
- Insights on patient burden/disease prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years
- Understand the existing market opportunities in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identifying strong upcoming players in the market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the conjoint analysis section to provide visibility around leading classes.
- Highlights of access and reimbursement policies of approved therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing market so that the upcoming players can strengthen their development and launch strategy.