Burns Market
- The incidence of burn injuries in the US is on the rise, driven by a surge in industrial accidents, household mishaps, and natural disasters.
- Greater awareness about the importance of prompt and appropriate treatment for burn injuries, coupled with improved access to healthcare services in the 7MM, is expected to drive demand for burn care products and therapies by 2034.
- The rise of telemedicine and remote monitoring in burn care is advancing post-discharge follow-up and long-term management. These technologies enable healthcare providers to track wound healing remotely, intervene promptly, and offer crucial patient education and support. As a result, their anticipated impact is poised to drive significant market advancement throughout the forecast period (2024-2034).
- The treatment of burns is based on both the location and severity of the damage. The severity of a burn is typically categorized into three main types: first-degree or superficial burns, second-degree or partial-thickness burns, and third-degree or full-thickness burns. The treatment approach for each type varies based on the depth of the burn and the affected skin layers.
- In the current standard treatment for skin burns, a combination of medications, wound dressings, therapy, skin grafting, and surgery is utilized to achieve several goals, including pain control, debridement of dead tissue, infection prevention, scar reduction, and restoration of function.
- Additionally, medical interventions encompass water-based therapies like ultrasound mist to cleanse and stimulate wound tissue, IV fluids to prevent dehydration and organ failure, analgesics such as morphine, anxiolytics, topical agents like bacitracin and silver sulfadiazine, and a variety of wound dressings and antimicrobials such as silver nitrate, maenad, bacitracin, and xeroform.
- In 2023, the market size of burns was highest in the US among the 7MM, accounting for approximately USD 854 million. With an aging population, there is an increased susceptibility to burn injuries globally due to factors such as decreased mobility, sensory impairments, and medical conditions that compromise skin integrity. This demographic trend is likely to drive demand for burn care products and services.
- Wound cleaning, pain control, surgical intervention, and scar treatment, including expensive skin grafting, notably autografts, underscore gaps in burn care, emphasizing unmet needs.
- Additionally, the provision of tailored psychological support services for burn survivors, effectively targeting their specific emotional and mental health obstacles, represents a significant challenge within burn care treatment.
DelveInsight’s “Burns Market Insights, Epidemiology, and Market Forecast – 2034” report delivers an in-depth understanding of burns, historical and forecasted epidemiology, as well as the burns market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
The burns market report provides current treatment practices, emerging drugs, market share of individual therapies, and current and forecasted 7MM burns market size from 2020 to 2034. The report also covers current burns treatment practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s potential.
Geography Covered
- The United States
- EU4 (Germany, France, Italy, and Spain) and the United Kingdom
- Japan
Study Period: 2020–2034
Burns Understanding and Treatment Algorithm
Burns Overview
A burn is an injury to the skin or other organic tissue primarily caused by heat or due to radiation, radioactivity, electricity, friction, or contact with chemicals, with associated significant morbidity and mortality. Burn damage triggers a complex pathophysiological response in the body. Initially, there is tissue damage characterized by cell death, disruption of the skin barrier, and inflammation. Severe burns can result in a systemic inflammatory response syndrome or even sepsis due to compromised immune function and increased susceptibility to infections. Of great importance is that the injury affects not only physical health but also the patient’s mental health and quality of life. Burn injuries are underappreciated trauma that can affect anyone, anytime and anywhere.
Burns Diagnosis
The determination of the severity of a burn depends on the depth of the burn and the width of the area. It is necessary to wait for 24–48 h to determine the exact burn grade, as the depth of the burn may increase due to edema and infection. The depth of the burn varies according to the type of the causative agent, the degree of temperature, and the thickness and vascularity of the affected skin area. Accurate assessment of the severity of a burn injury is paramount because it forms the basis for all subsequent treatment decisions, triage plans, and assessment of medical futility. Whenever possible, decisions about proceeding after diagnosis and screening should incorporate patient preferences and expectations about the quality of life. Optimal assessment of the severity of burn injury must involve a systematic, methodical approach, such as that described in course materials for the Advanced Trauma Life Support (ATLS) by the American College of Surgeons Committee on Trauma, Emergency Management of the Severe Burn (EMSB) by the Australian and New Zealand Burn Association, and Advanced Burn Life Support (ABLS) by the ABA.
Further details related to country-based variations are provided in the report…
Burns Treatment
The current treatment for skin burns involves medications, wound dressings, therapy, and surgery. The treatment goals are to control pain, remove dead tissue, prevent infection, reduce scarring risk, and regain function. The treatment depends on the severity of the burn.
Medical treatment includes water-based treatments (Ultrasound mist therapy to clean and stimulate the wound tissue), fluids to prevent dehydration (intravenous (IV) fluids to prevent dehydration and organ failure), pain and anxiety medications (morphine and anti-anxiety medications), creams and ointments (bacitracin and silver sulfadiazine), dressings (wound dressings), and common topical antimicrobials such as silver sulfadiazine (SSD), cerium plus SSD, silver nitrate, mafenide, bacitracin, and Xeroform. Apart from the above-mentioned pharmacological treatment options, the most important management practice involves surgery. Early autografting then builds on these improvements by rapidly closing excised wounds, further reducing infection risk, decreasing pain, and enabling earlier mobilization. If there are concerns about the viability or bacterial load on the wound bed and/or the patient’s stability, allografting is preferred to cover the debrided wound temporarily. Some commercially available skin substitutes are Epicel, StrataGraft, RECELL System, Split thickness skin graft, Kerecis Omega3 products, and several others.
Burns Epidemiology
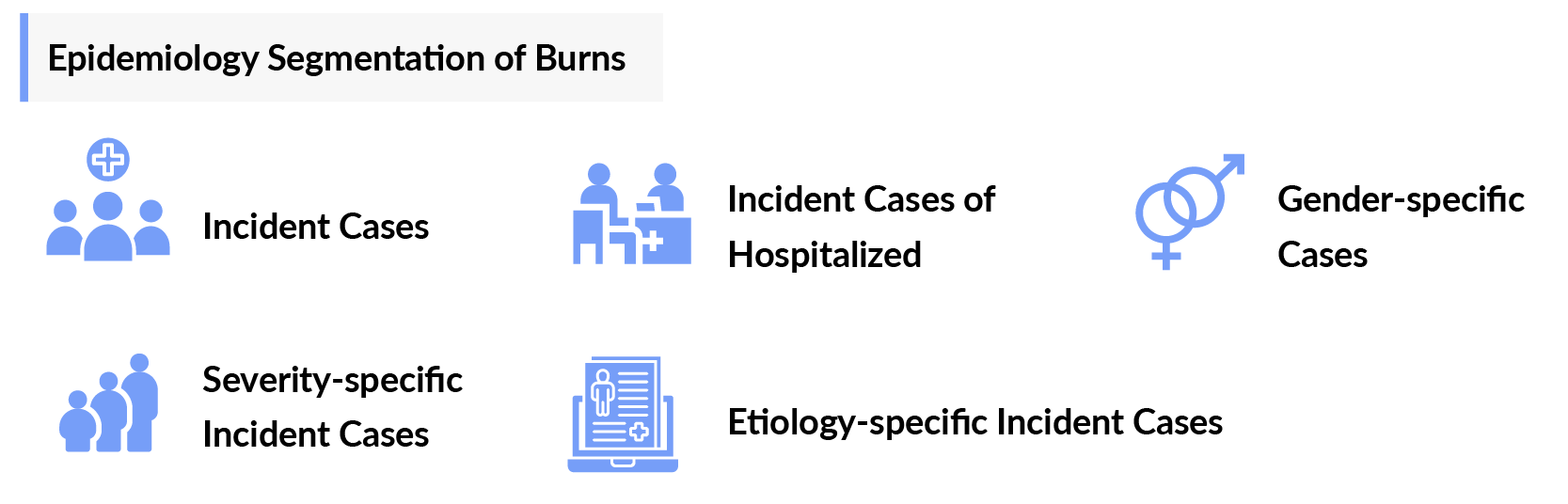
As the market is derived using a patient-based model, the burns epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by incident cases of burn patients requiring treatment, gender-specific incident cases of treated burn injuries, etiology-specific incident cases of treated burn injuries, severity-specific incident cases of treated burn injuries, and incident cases of hospitalized burn patients in the 7MM covering the United States, EU4 countries (Germany, France, Italy, and Spain) and the United Kingdom, and Japan from 2020 to 2034.
- As per DeveInsight analysis, in 2023, the total incident cases of burn injuries were reported to be approximately 5.4 million cases, out of which nearly 1.8 million cases required medical treatment in the 7MM. These cases are expected to increase by 2034, driven by a surge in industrial accidents, household mishaps, and natural disasters.
- The number of hospitalized burn cases in the 7MM market is expected to increase at an effective CAGR of 0.54% during the study period, i.e. 2020–2034. The forecast period is expected to see a rise in these cases, propelled by heightened awareness of the critical need for timely and suitable treatment of burn injuries, which in turn motivates patients to seek medical care for such cases.
- In 2023, the US had the highest incident population of burn injuries that required medical treatment with nearly 671,128 cases followed by Japan with approximately 329,241 cases and the UK with 280,817 cases.
- Among the EU4 and the UK, the UK had the highest incident population of burn patients that require medical treatment accounting for approximately 280,817 cases, followed by France and Spain with nearly 204,506, and 145,510 cases respectively.
- According to DeveInsight analysis in the EU4 and the UK, the majority of burn injury cases taking treatment were males with approximately 540,480 cases and 341,627 female cases in 2023. This is accompanied by the fact that major burn injuries occur at workplaces due to various occupational hazards and exposures.
- Based on etiology-specific burn incident cases in the US, flame/fire was the foremost contributing cause for burn injuries accounting for approximately 41%, while chemical contact occupied the least share with nearly 3.5% in 2023. Anticipated increases in such cases are attributed to activities such as cooking, motor vehicle incidents, and outdoor pursuits like camping or bonfires.
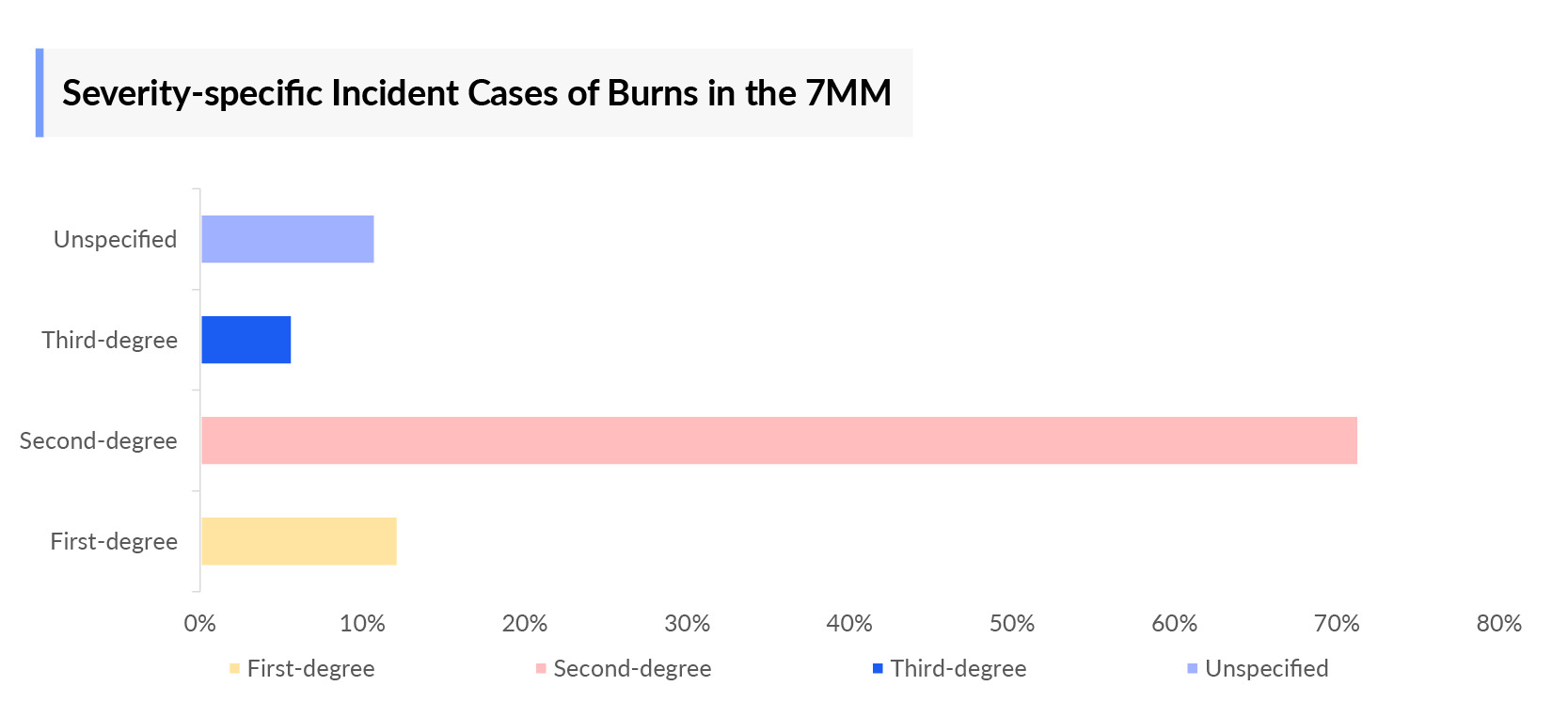
- In 2023, the severity-specific incident cases were approximately 11,483; 759,667; 79,500; and 32,683 for first-degree, second-degree, third-degree, and unspecified, respectively in the EU4 and the UK.
- Japan accounted for nearly 17.5% of incident cases of burn injuries requiring medical treatment in 2023. Whereas, the severity-specific incident cases were approximately 71,774; 192,277; 7,243; and 57,946 for first-degree, second-degree, third-degree, and unspecified, respectively in 2023. The forecast period is likely to witness an increase in such cases, driven by Japan's aging population, which is more susceptible to accidents at home, such as burns from cooking or scalding water.
Burns Drug Chapters
The drug chapter segment of the burns report encloses a detailed analysis of burns-marketed drugs and late-stage (Phase III and Phase II) pipeline drugs. It also helps understand the burns clinical trial details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug, and the latest news and press releases.
Marketed Drugs
NexoBrid/KMW-1: MediWound Germany GmbH/Kaken Pharmaceutical
NexoBrid, a concentrate of proteolytic enzymes enriched in bromelain, is an easy-to-use, topically-applied product that removes eschar in 4 h without harming the surrounding healthy tissues. NexoBrid’s rapid and selective debridement alleviates the known risks associated with eschars, such as infection, eventual sepsis, wound deterioration, and consequential scarring. It allows physicians to reach an informed decision on further treatment at an earlier stage by direct visual assessment of the actual burn depth. Furthermore, it minimizes the burden associated with invasive surgical procedures, reduces the need for skin grafting and sacrifice of healthy tissue from donor sites on a patient’s body, and generally results in a more favorable overall long-term patient outcome NexoBrid is approved in over 40 countries including the United States, European Union, and Japan.
Kerecis Omega3: Kerecis
Kerecis (formally known as Marigen) produces skin and tissue-based products for use in surgery and for treating wounds. Kerecis Omega3 is intact fish skin rich in naturally occurring Omega3 polyunsaturated fatty acids. The material, which is used to regenerate damaged human tissue, has the potential to accelerate healing. When grafted onto damaged human tissue such as a burn or a diabetic wound, the material recruits the body’s own cells and is ultimately converted into living tissue. Kerecis has a wide range of products for the treatment of burns that includes Kerecis Omega3 Wound, Kerecis Omega3 OR, Kerecis Omega3 MicroGraft, Kerecis Omega3 GraftGuide, and Kerecis Primary Wound Spray, some of which are approved and some are not. Kerecis products utilize multiple patented fatty-acid technologies to protect and regenerate tissue across two core technologies: Kerecis Omega3 Fish Skin and Kerecis Omega3 Viruxid.
StrataGraft: Mallinckrodt Pharmaceuticals
StrataGraft is a viable, bioengineered, allogeneic, cellularized scaffold product derived from keratinocytes grown on gelled collagen-containing dermal fibroblasts. It is designed to deliver viable cells to support the body’s ability to heal and contains metabolically active cells that produce and secrete a variety of growth factors and cytokines. Growth factors and cytokines are known to be involved in wound repair and regeneration. The product is designed with both dermal and epidermal layers composed of well-characterized human cells. StrataGraft is intended to be applied in appropriate aseptic conditions, such as the operating room, and can be sutured, stapled, or secured with a tissue adhesive. In June 2021, the US FDA approved Mallinckrodt Pharmaceuticals StrataGraft (allogeneic cultured keratinocytes and dermal fibroblasts in murine collagen – dsat) for the treatment of adults with thermal burns containing intact dermal elements for which surgical intervention is clinically indicated (deep partial-thickness burns).
Note: Further marketed drugs and their details will be provided in the report…
Emerging Drugs
MW-III: Skingenix
MW-III, a product by Skingenix, is a topical product being evaluated for second-degree burns in adult patients. The drug is a botanical drug product developed for treating burns and other cutaneous wounds, which will be made from natural ingredients such as Sesame Oil, Coptidis Rhizoma, Scutellariae Radix, Phellodendri Chinesis Cortex, Pheretima, formulated as an ointment with Beeswax.
The drug candidate is being investigated to assess the safety and efficacy of treating patients with burn injuries, currently in a Phase II trial expected to be completed in December 2024.
DenovoSkin (EHSG-KF): CUTISS AG
DenovoSkin being developed by CUTISS AG and others, is bio-engineered within large quantities (>1:100) with a thick, double-layer, robust structure. DenovoSkin is a patented, personalized, autologous bio-engineered human skin graft classified as an Advanced Therapy Medical Product (ATMP). DenovoSkin has completed Phase I in pediatric patients. The company is working towards bringing this promising treatment to patients suffering from severe burns and skin injuries and is also working on the scale-up of the production process using automation. The drug candidate is currently undergoing evaluation in two Phase II/III trials, and comprehensive data analysis, including a three-year follow-up, is expected to be available by 2025.
Note: Further emerging therapies and their detailed assessment will be provided in the final report….
Drug Class Insights
The treatment of burns involves various medications and ointments, such as Albumin human, Cinchocaine, Mafenide Acetate, Nitrofurazone, Silver Sulfadiazine, Sodium Hyaluronate, and Zinc oxide, among others. These are utilized to address shock, alleviate pain and itching, and prevent bacterial infections commonly associated with burn injuries.
The current promising pharmacological classes for burns treatment include topical antimicrobial agents, analgesics, corticosteroids, NSAIDs, growth factors and cytokines, biological skin substitutes, and others.
Topical antimicrobial treatments, including silver sulfadiazine, mafenide acetate, and silver nanoparticles, serve to prevent and treat infections in burn injuries. Analgesics such as acetaminophen, ibuprofen, and opioids are administered for pain management, while corticosteroids and NSAIDs help alleviate inflammation and swelling. Additionally, silver-containing dressings, hydrogels, and collagen-based dressings, along with other topical agents, facilitate wound healing and tissue regeneration. In severe cases, recombinant human growth factors and granulocyte colony-stimulating factor (G-CSF) may be employed to support tissue repair and regeneration. Furthermore, cultured epithelial autografts and dermal substitutes, are also used to promote wound healing and improve outcomes in severe burn injuries.
Burns Market Outlook
In the current market scenario, the standard treatment for skin burns involves medications, wound dressings, therapy, and surgery. The treatment goals are to control pain, remove dead tissue, prevent infection, reduce scarring risk, and regain function. The treatment depends on the severity of the burn.
Apart from the pharmacological treatment options, the most important management practice involves surgery. Early autografting then builds on these improvements by rapidly closing excised wounds, further reducing infection risk, decreasing pain, and enabling earlier mobilization. If there are concerns about the viability or bacterial load on the wound bed and/or the patient’s stability, allografting is preferred to cover the debrided wound temporarily.
The surgical approach is to leave no full-thickness burned tissue behind and debride down to viable tissue. The gold-standard burn coverage is autologous split-thickness skin grafts (STSGs). Skin substitutes have developed over the past decade from temporary materials used to induce wound healing to permanent tissue-engineered materials that offer definitive healing.
The common principle of skin substitutes is to deliver proteins, growth factors, and/or cells via a delivery vehicle or matrix that will then be integrated into the wound and form new autologous skin. Common skin substitutes include cadaver skin (allograft) and porcine skin (xenograft), which provide temporary coverage for up to 14 days before inevitable rejection. To overcome the need for uninjured donor skin in autologous STSG, artificial skin substitutes have been introduced to the market and include TransCyte, CEA, cultured skin substitutes, and various others.
- The current market has been segmented into different commonly used therapeutic classes based on the prevailing treatment pattern across the 7MM, which presents minor variations in the overall prescription pattern. NexoBrid/KMW-1, Kerecis Omega3 products, RECELL System, and others are the major drugs covered in the forecast model.
- Key players including DenovoSkin (EHSG-KF), MW-III, realSKIN (Xeno-Skin), and others are being evaluated in different stages of clinical development. They aim to investigate their products for the treatment of burns.
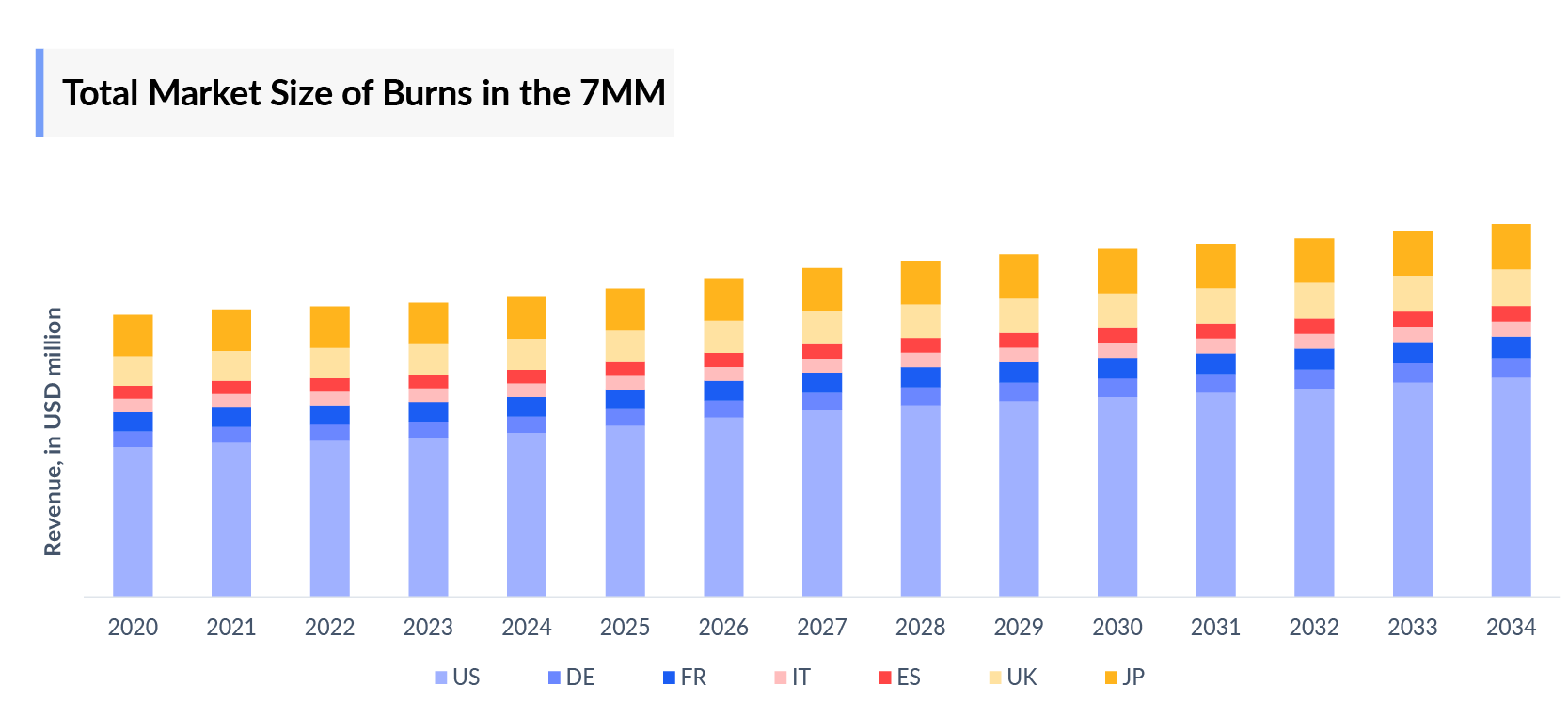
- In 2023, the total market size of burns in the 7MM was approximately USD 1,577 million, out of which the US accounted for approximately USD 854 million.
- DelveInsight’s analysts estimate that the market is expected to show positive growth, mainly attributed to the increase in population, increasing incidence of burn injury and also, and the launch of upcoming therapies during the forecast period (2024-2034).
- The Burns market size in the EU4 and the UK was nearly USD 501 million and accounted for nearly 32% of the total 7MM market size in 2023.
- Among the EU4 and the UK, the United Kingdom holds the highest market size of around USD 165 million followed by France, and Germany with approximately USD 104 million, and USD 87 million respectively. These numbers are expected to change during the forecast period.
- The burns market size in Japan accounted for nearly 14% of the total 7MM market size in 2023, these numbers are expected to change by 2034.
Continued in report…
Burns Drugs Uptake
This section focuses on the uptake rate of potential drugs expected to be launched in the market during 2020–2034. For example, denovoSkin (EHSG-KF), a bio-engineered human skin graft classified as an advanced therapy medical product, is projected to enter EU4 and the UK in 2027 and is predicted to have a slow-medium uptake during the forecast period.
Further detailed analysis of emerging therapies drug uptake in the report…
Burns Pipeline Development Activities
The report provides insights into different therapeutic candidates in Phase III, Phase II, and Phase I. It also analyzes key players involved in developing targeted therapeutics.
Pipeline development activities
The report covers information on collaborations, acquisitions and mergers, licensing, and patent details for emerging therapies for burns.
KOL Views
To keep up with current market trends, we take KOLs and SMEs’ opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts contacted for insights on burns evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake, along with challenges related to accessibility, including Medical/scientific writers, Medical Professionals, Professors, Directors, and Others.
DelveInsight’s analysts connected with 50+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. Centers like the USGS Northern Rocky Mountain Science Center, Department of Plastic and Reconstructive Surgery, Germany, Clocheville Pediatric Hospital, France, University of Bari, Italy, and Bristol Royal Hospital for Children, UK were contacted. Their opinion helps understand and validate current and emerging therapy treatment patterns or burns market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
Physician’s View
According to our primary research analysis, despite considerable progress in burn treatment, there persists a significant unmet need. One of the primary challenges lies is the lack of affordable access to advanced skin grafting techniques, and comprehensive psychological support services tailored to burn survivors' long-term well-being. Thus, physicians emphasize the urgent need for improved burn treatment options, including strategies to accelerate wound healing, mitigate hypermetabolic-catabolic conditions, control wound infections, and reduce overall recovery time. Their perspectives underscore the necessity for innovative wound care modalities, such as advanced dressings and regenerative therapies, to promote faster tissue regeneration. Additionally, they advocate for targeted interventions to address metabolic imbalances, novel antimicrobial agents to combat infections, and comprehensive, multidisciplinary approaches to burn care that integrate personalized rehabilitation and psychosocial support.
According to a KOL in the US, There are several devastating consequences of burns, including significant morbidity, emotional distress, and a reduced quality of life. In addition to immediate care, burns frequently require long-term treatment that requires frequent outpatient visits (dressing changes, etc.) and multiple reconstructive surgical procedures while in the hospital.
As per another KOL, the professional treatment of burn injuries caused by fire, scalds, explosions, chemicals, or electricity places high demands on medical professionals. This does not only apply to severe burn injuries. Small burns on the face or hands are easily underestimated by laypersons or less experienced doctors since deeper injuries often look relatively harmless on the surface
In another KOL in Italy, first aid can be provided in a conventional, non-specialized hospital. However, if the patient's burn is judged to be very deep by the doctors on arrival, he will be very quickly referred to a burns treatment center.
The current pipeline contains Dermo-epidermal skin grafts, stem cells, plant extracts, and several others that target regenerative cell therapy in treating burns. The entry of these drugs will provide different options relating to patient-specific needs.
Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT analysis and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Conjoint Analysis analyzes multiple emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
In efficacy, the trial’s primary and secondary outcome measures are evaluated; for instance, in burns trials, one of the most important primary outcome measures is complete eschar removal.
Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the route of administration, order of entry and designation, probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Market Access and Reimbursement
The high expenses associated with burn treatment therapies pose a significant obstacle to the growth of the pharmaceutical market, as patients may forego proper treatment due to financial constraints. Reimbursement issues compound the challenge, with medical care for burns often necessitating specialized attention and covering various aspects like diagnosis, treatment, and ongoing support. Limited coverage by health insurance plans for specific therapies and treatments can lead to substantial out-of-pocket costs for families seeking optimal care. Additionally, accessing specialized healthcare providers proficient in burn care may be difficult, and insurance coverage may not always fully offset associated expenses.
The ACell Reimbursement Support Center – supported by The Pinnacle Health Group – is available to assist with questions for all ACell products, including Cytal Burn Matrix. Reimbursement and eligibility for coverage for the use of these products and associated procedures vary by Medicare and payers. The coverage allows to research coverage policy information for ACell products, access ACell product reference tools, and review inadequate reimbursements. Coverage policies, prior authorizations, contract terms, billing edits, and site of service influence reimbursement. However, it is recommended that providers verify coverage and billing policies.
Kerecis Omega3 Wound is reimbursed by Medicare in the high-cost group for applications of skin substitutes in the HOPD setting using CPT codes 15271-15278. Medicare payment for Q4158 – Kerecis Omega3 Wound is included in the payment for the application. In February 2019, Kerecis received a notice from Swiss healthcare authorities that the company’s lead product, Kerecis Omega3 Wound, will be reimbursed in Switzerland. The product has a Medicare “Q” code (Q4158), allowing it to be easily identified and processed by Medicare and private insurance companies
The report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenarios, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Further details will be provided in the report…
Scope of the Report
- The report covers a segment of key events, an executive summary, descriptive overview of burns, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight has been provided into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, disease progression, and treatment guidelines.
- Additionally, an all-inclusive account of the current and emerging therapies, along with the elaborative profiles of late-stage and prominent therapies, will impact the current treatment landscape.
- A detailed review of the burns market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help shape and drive the 7MM burns market.
Burns Report Insights
- Patient Population
- Therapeutic Approaches
- Burns Pipeline Analysis
- Burns Market Size and Trends
- Existing and Future Market Opportunity
Burns Report Key Strengths
- 11 years Forecast
- The 7MM Coverage
- Burns Epidemiology Segmentation
- Key Cross Competition
- Attribute analysis
- Drugs Uptake and Key Market Forecast Assumptions
Burns Report Assessment
- Current Treatment Practices
- Unmet Needs
- Pipeline Product Profiles
- Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
Key Questions
Burns Market Insights
- What was the burns market share (%) distribution in 2020 and what it would look like in 2034?
- What was the total market size of burns by therapies, and market share (%) distribution in 2020, and what would it look like by 2034? What are the contributing factors for this growth?
- How will DenovoSkin (EHSG-KF), and MW-III affect the treatment paradigm of burns?
- How will DenovoSkin (EHSG-KF) compete with upcoming products and marketed therapies?
- Which drug is going to be the largest contributor by 2034?
- What are the pricing variations among different geographies for approved and marketed therapies?
- How would future opportunities affect the market dynamics and subsequent analysis of the associated trends?
Burns Epidemiology Insights
- What are the disease risks, burdens, and unmet needs of burns? What will be the growth opportunities across the 7MM concerning the patient population with burns?
- What is the historical and forecasted burn patient pool in the United States, EU4 (Germany, France, Italy, and Spain) the United Kingdom, and Japan?
- Out of the above-mentioned countries, which country would have the highest diagnosed prevalent burns population during the forecast period (2023–2034)?
- What factors are factors contributing to the growth of burn cases?
Current Treatment Scenario, Marketed Drugs, and Emerging Therapies
- What are the current options for the treatment of burns? What are the current guidelines for treating burns in the US and Europe?
- How many companies are developing therapies for the treatment of burns?
- How many emerging therapies are in the mid-stage and late stage of development for treating burns?
- What are the recent novel therapies, targets, mechanisms of action, and technologies developed to overcome the limitations of existing therapies?
- What is the cost burden of current treatment on the patient?
- Patient acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the country-specific accessibility issues of approved therapies?
- What is the 7MM historical and forecasted market of burns?
Reasons to Buy
- The report will help develop business strategies by understanding the latest trends and changing treatment dynamics driving the burns market.
- Insights on patient burden/disease prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- Understand the existing market opportunities in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data in the US, EU4 (Germany, France, Italy, and Spain) the United Kingdom, and Japan.
- Identifying strong upcoming players in the market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the conjoint analysis section to provide visibility around leading classes.
- Highlights of access and reimbursement policies for stuttering, barriers to accessibility of approved therapy, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing market so that the upcoming players can strengthen their development and launch strategy.