Cancer Cachexia Market Summary
- The Cancer Cachexia market in the 7MM was USD 483 million in 2025 and projected to reach USD 1038 million by 2034.
- The Cancer Cachexia market is projected to grow at a CAGR of 8.9% by 2034 in leading countries (US, EU4, UK and Japan).
Cancer Cachexia Market and Epidemiology Analysis
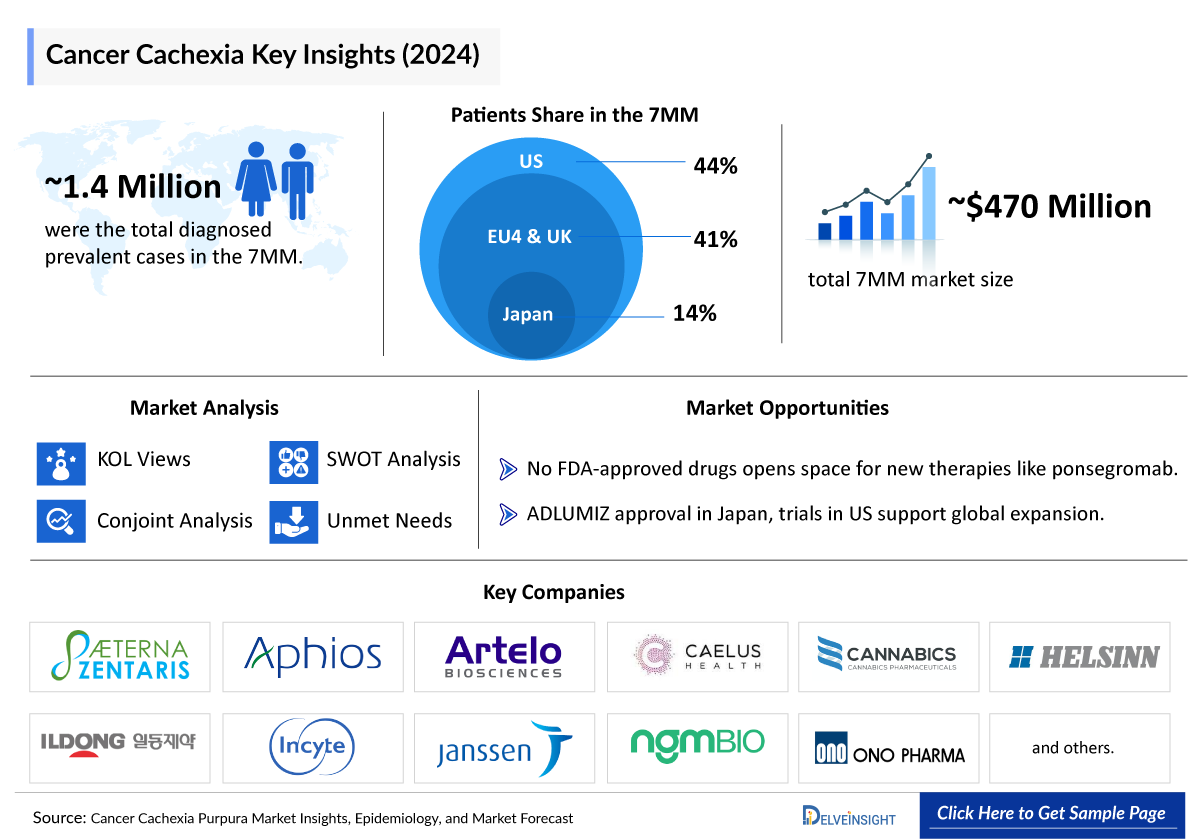
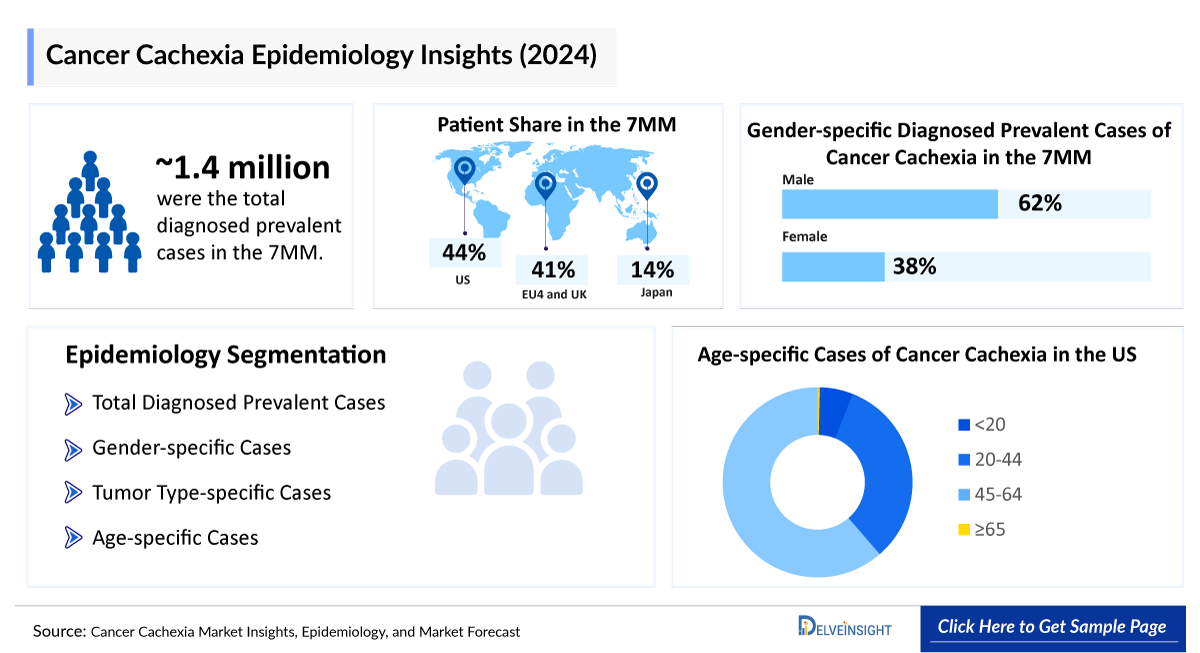
- According to DelveInsight’s estimates, in 2024, there were approximately 1.4 million diagnosed prevalent cases of cancer cachexia in the 7MM. Of these, the US accounted for nearly 44.0% of the cases, while the EU4 and the UK accounted for around 40.5% and Japan represented approximately 15.5% of the cases, respectively.
- The cancer cachexia market is projected to see consistent growth, with a robust Compound Annual Growth Rate (CAGR) anticipated from 2025 to 2034. This expansion across the 7MM will be driven by the introduction of innovative therapies, ponsegromab (PF-06946860), S-pindolol benzoate (ACM-001.1), and TCMCB07, among others. Additionally, the rising prevalence of cancer cachexia—driven by factors such as an aging population, improved cancer survival rates that allow more patients to develop cachexia, increasing incidence of high-risk tumor types (e.g., lung and pancreatic cancers), and enhanced diagnostic awareness—continues to challenge clinical management.
- According to DelveInsight’s analysis, the cancer cachexia market in the 7MM was valued at approximately USD 475 million in 2024. Over the forecast period from 2025 to 2034, this market is projected to grow at a CAGR of 8.9%.
- Current management combines dietary counseling, selective enteral or parenteral feeding, and off-label pharmacotherapy (megestrol acetate, corticosteroids, low-dose olanzapine). These interventions produce transient appetite stimulation and predominantly fat gain, with inconsistent effects on lean body mass or survival and notable toxicity concerns.
- No US FDA-approved therapies exist, and regulatory approval is limited to Japan’s ADLUMIZ; the European Medicines Agency (EMA) issued a negative opinion due to marginal lean body mass efficacy, lack of functional or quality-of-life benefits, and safety data gaps. This paucity of approved, disease-modifying options underscores an urgent need for agents that preserve muscle mass, enhance function, and improve survival.
- Pfizer, Actimed Therapeutics, and Endevica Bio, among others are progressing their assets through various clinical trial phases, driving innovation in the cancer cachexia market and creating significant growth opportunities.
- Pfizer’s ponsegromab (PF-06946860), set to launch in the US in 2029, is projected to become the top-selling drug by 2034, highlighting strong market uptake and its potential to address significant unmet needs.
Cancer Cachexia Market size and forecast
- 2025 Sleep Apnea Market Size: USD 483 million
- 2034 Projected Sleep Apnea Market Size: USD 1038 million
- Sleep Apnea Growth Rate (2025-2034): 8.9% CAGR
- Largest Spinal Cord Injury Market: United States
DelveInsight’s “Cancer Cachexia – Market Insights, Epidemiology, and Market Forecast – 2034” report delivers an in-depth understanding of cancer cachexia, historical and forecasted epidemiology, as well as the cancer cachexia market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
The cancer cachexia market report provides current treatment practices, emerging drugs, market share of individual therapies, and current and forecasted 7MM cancer cachexia market size from 2020 to 2034. The report also covers cancer cachexia treatment practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s potential.
Scope of the Cancer Cachexia Market | |
|
Study Period |
2020–2034 |
|
Forecast Period |
2025–2034 |
|
Geographies Covered |
US, EU4 (Germany, France, Italy, and Spain) and the UK, and Japan |
|
Cancer Cachexia Epidemiology |
|
|
Cancer Cachexia Market |
|
|
Market Analysis |
|
|
Cancer Cachexia Market players |
|
|
Future opportunity |
An evolving landscape of longer cancer survivorship and growing recognition of cachexia’s impact is driving demand for targeted, disease‑modifying therapies. The absence of approved options in major markets highlights a clear unmet need that late‑stage candidates—such as GDF‑15 inhibitors and multimodal Anabolic/Catabolic Transforming Agents—are well positioned to address. Success will depend on strategic biomarker‑guided patient selection, innovative combination regimens that tackle inflammatory and metabolic drivers simultaneously, and efficient regulatory navigation outside Japan’s current approval. Collaborative partnerships across industry and academia will be critical to accelerate development and secure market access, ultimately transforming cachexia care. |
Factors impacting the Cancer Cachexia Market Growth
Rising Cancer Prevalence and Larger Eligible Patient Pool
More cancer diagnoses, plus improved survival for many tumor types, increase the number of patients who either develop or survive long enough to experience cancer-related wasting. In 2024, DelveInsight estimated approximately 1.4 million diagnosed prevalent cases of cancer cachexia across the 7MM, reflecting a significant burden on global healthcare systems. That growing patient base directly expands demand for effective cachexia interventions and supportive-care services.
ADLUMIZ’s Role in Shaping Cancer Cachexia Market
The approval of ADLUMIZ for multiple cancer types in Japan and its ongoing evaluation in the US present a strategic opportunity to expand therapeutic options for cancer cachexia across major markets.
Emerging Cancer Cachexia Therapeutic Opportunities
The lack of US FDA-approved treatments presents a significant opportunity for emerging therapies like Ponsegromab (Pfizer), S-pindolol benzoate (Actimed Therapeutics), TCMCB07 (Endevica Bio), Rilogrotug (AVEO Oncology), and others to fill the unmet need in managing cancer cachexia.
Cancer Cachexia Disease Understanding
Cancer cachexia overview
Cancer cachexia is a complex wasting syndrome seen in advanced cancers, marked by progressive loss of skeletal muscle and fat, leading to severe weakness. Unlike simple malnutrition, it cannot be reversed by nutrition alone. The condition is driven by systemic inflammation, primarily mediated by cytokines (signaling proteins released by tumors and host cells). These cytokines disrupt metabolism, causing anorexia (loss of appetite), insulin resistance (impaired glucose uptake), and a shift toward catabolism (tissue breakdown) over anabolism (tissue building). This creates a vicious cycle of energy deficit, particularly in aggressive cancers like pancreatic, lung, and head/neck malignancies.
Clinically, cancer cachexia presents with weight loss, fatigue, anemia, and hormonal imbalances, leading to a visibly emaciated appearance. Pain often coexists due to shared inflammatory pathways, such as elevated Interleukin-6 (IL-6) and C-reactive Protein (CRP), worsening muscle loss and reducing mobility. The syndrome progresses through stages: pre-cachexia (early metabolic changes), cachexia (significant weight loss), and refractory cachexia (severe functional decline with poor survival). These stages reflect worsening metabolic dysfunction, ultimately leading to complications like organ failure and severely diminished Quality of Life (QoL).
Cancer cachexia diagnosis
Diagnosing cancer cachexia requires a multipronged approach due to the lack of a single definitive test. Key assessments include nutritional screening (using tools like the Patient-Generated Subjective Global Assessment [PG-SGA] or Mini Nutritional Assessment [MNA]), body composition analysis (via computed tomography [CT], the gold standard, or dual-energy X-ray absorptiometry [DXA] and bioelectrical impedance analysis [BIA]), and muscle function tests (e.g., handgrip strength). Biomarkers like low albumin and high CRP support diagnosis but are nonspecific. Integrated scoring systems, such as the modified Glasgow Prognostic Score (mGPS) and Cancer Cachexia Score (CASCO), help stage the disease and predict outcomes by combining clinical, metabolic, and functional data.
Further details related to country-based variations are provided in the report…
Cancer cachexia treatment
Management of cancer cachexia involves a multidisciplinary strategy. Nutritional support focuses on high-protein, high-calorie diets, with enteral or parenteral nutrition reserved for specific cases. Pharmacologic options include corticosteroids (short-term use) and progesterone analogs (e.g., megestrol acetate) for appetite stimulation. Low-dose olanzapine has shown promise in improving appetite during chemotherapy. Resistance training may help preserve muscle, while psychological support addresses distress and maintains QoL. However, no single therapy is universally effective, emphasizing the need for personalized, integrated care targeting inflammation, metabolism, and symptom control.
Cancer Cachexia Epidemiology
As the market is derived using a patient-based model, the cancer cachexia epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented total diagnosed prevalent cases of cancer cachexia, gender-specific cases of cancer cachexia, tumor type-specific cases of cancer cachexia, and age-specific cases of cancer cachexia in the 7MM covering the United States, EU4 countries (Germany, France, Italy, and Spain) and the United Kingdom, and Japan from 2020 to 2034.
Key Findings from Cancer Cachexia Epidemiological Analyses and Forecast
- According to DelveInsight’s estimates, the total diagnosed prevalent cases of cancer cachexia in the 7MM were around 1.4 million in 2024 which are expected to rise by 2034.
- Among the 7MM, the US accounted for nearly 44.0% of the total diagnosed prevalent cases of cancer cachexia, with around 610 thousand cases in 2024. These cases are expected to increase during the forecast period (2025–2034).
- In 2024, among EU4 and the UK, Germany reported the highest number of diagnosed prevalent cases of cancer cachexia, with approximately 145 thousand cases, whereas Spain had the lowest cases, around 80 thousand.
- In 2024, Japan reported approximately 215 thousand diagnosed prevalent cases of cancer cachexia, a figure expected to rise by 2034.
- DelveInsight estimates that cancer cachexia shows a notable male predominance. In the US, diagnosed prevalent cases in 2024 were distributed as 60% among males and 40% among females.
- According to DelveInsight, among the EU4 and the UK, colorectal cancer accounted for the highest proportion of cancer cachexia cases at approximately 22%, while melanoma of the skin represented the lowest share at nearly 2%.
- According to DelveInsight’s age-specific prevalence estimates, in 2024, Japan reported approximately 323 cases of cancer cachexia in individuals aged under 20, 9 thousand cases in the 20–44 age group, 65 thousand cases in those aged 45–64, and 140 thousand cases in the 65 and older age group. These cases are expected to increase by 2034.
Cancer Cachexia Epidemiology Segmentation
- Total Diagnosed Prevalent Cases of Cancer Cachexia
- Gender-specific Cases of Cancer Cachexia
- Tumor type-specific Cases of Cancer Cachexia
- Age-specific Cases of Cancer Cachexia
Cancer Cachexia Drug Chapters
The drug chapter segment of the cancer cachexia report encloses a detailed analysis of cancer cachexia marketed drugs and mid to late-stage (Phase III and Phase II) pipeline drugs. It also helps understand the cancer cachexia clinical trial details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug and the latest news and press releases.
Cancer Cachexia Marketed Drugs
ADLUMIZ (anamorelin hydrochloride): Helsinn Healthcare/Ono Pharmaceutical
ADLUMIZ is the first and only treatment approved in Japan for cancer cachexia, indicated for patients with weight loss and anorexia associated with non-small cell lung, gastric, pancreatic, and colorectal cancers. It addresses a significant unmet need by offering a fast-acting, sustained solution that improves appetite, body weight, and overall clinical condition.
Helsinn Healthcare is conducting a Phase II trial in the US to evaluate anamorelin for the prevention of cancer-related weight loss and anorexia in patients starting first-line treatment for advanced pancreatic cancer.
In January 2021, ADLUMIZ was approved in Japan for cancer cachexia associated with non-small cell lung cancer (NSCLC), gastric, pancreatic, and colorectal cancers, based on Phase II and III studies conducted locally. The approval was granted to Ono Pharmaceutical in collaboration with Helsinn Group. However, in the EU, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) issued a negative opinion in May 2017 recommending refusal of marketing authorization for the treatment of anorexia, cachexia, or weight loss in patients with NSCLC. This decision was upheld following a re-examination requested by Helsinn Birex Pharmaceuticals in September 2017.
Comparison of Cancer Cachexia Marketed Drugs | |||
|
Drug |
MoA |
RoA |
Company |
|
ADLUMIZ (anamorelin hydrochloride) |
Ghrelin receptor agonist |
Oral |
Helsinn Healthcare/Ono Pharmaceutical |
Cancer Cachexia Emerging Drugs
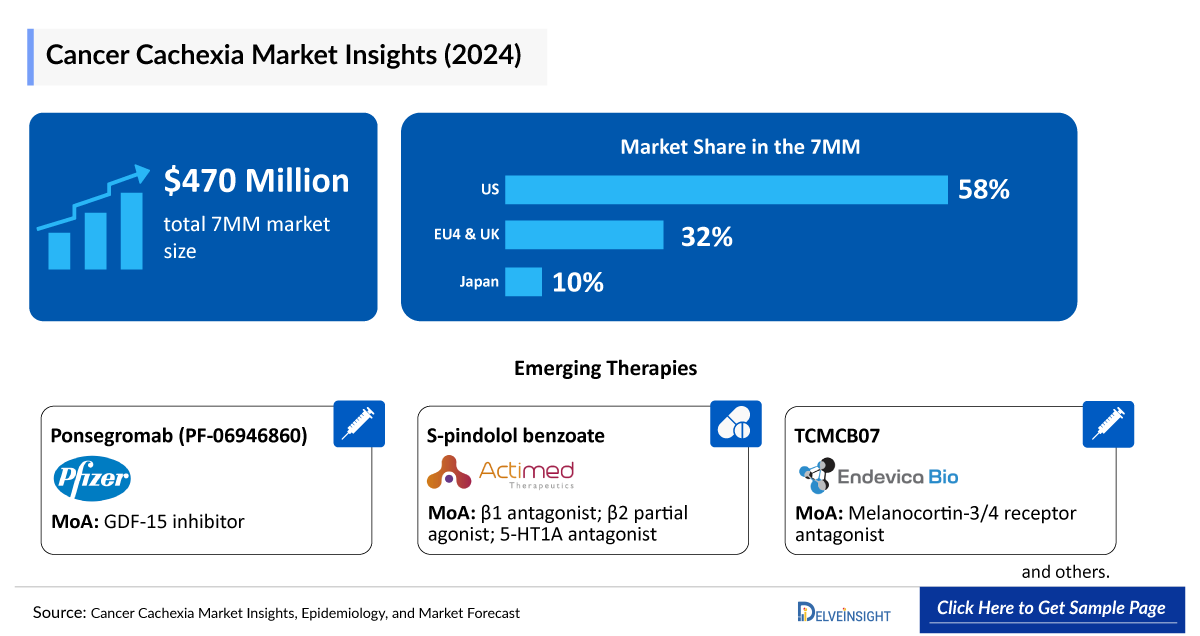
Ponsegromab (PF-06946860): Pfizer
Ponsegromab (PF-06946860) is an investigational monoclonal antibody being developed for the treatment of cancer cachexia by targeting Growth Differentiation Factor-15 (GDF-15). In a prior Phase Ib study, ponsegromab demonstrated proof of mechanism through marked suppression of unbound circulating GDF-15 levels. The trial also showed promising efficacy signals, including gains in body weight and improvements in patient-reported outcomes.
In September 2024, Pfizer reported positive Phase II results for ponsegromab in cancer cachexia, with registration studies expected to start in the second half of 2025.
S-pindolol benzoate (ACM-001.1; MT-102): Actimed Therapeutics
S-pindolol benzoate (ACM-001.1), developed by Actimed, is a first-in-class Anabolic/Catabolic Transforming Agent (ACTA) being advanced for the treatment of cancer cachexia. It acts through a unique multimodal mechanism involving ß1 receptor antagonism (reducing catabolism), partial ß2 receptor agonism (promoting anabolism), and central 5-hydroxytryptamine 1a (5-HT1a) antagonism (improving appetite and fatigue). A Phase I pharmacokinetic and pharmacodynamic study confirmed bioequivalence to racemic pindolol and the absence of in vivo racemization.
Akamis Bio, in collaboration with Veeda Oncology, conducted the Phase IIa ACT-ONE trial, where S-pindolol demonstrated proof-of-concept in cancer cachexia.
This led to the development of a new salt formulation, S-pindolol benzoate (ACM-001.1), which was evaluated in a Phase I study in healthy volunteers. Based on these findings, the company is now planning the IMPACT (IMProving Cancer Cachexia with ACTAs) Phase IIb/III program in patients with NSCLC and colorectal cancer, subject to securing Series B funding.
TCMCB07: Endevica Bio
TCMCB07 is a melanocortin-3/4 receptor antagonist peptide in clinical development for the treatment of cachexia. Designed as a first-in-class agent, it can cross the blood–brain barrier to target central receptors previously considered inaccessible, thereby modulating behavioral and metabolic responses to chronic disease. In preclinical models, TCMCB07 has consistently demonstrated the ability to preserve lean muscle mass and reverse cachexia across various underlying chronic conditions.
In April 2025, Endevica Bio initiated patient dosing in a Phase II trial of TCMCB07 (B07), evaluating its potential to prevent weight loss in patients with Stage IV metastatic colorectal cancer undergoing chemotherapy.
Comparison of Cancer Cachexia Emerging Drugs | ||||
|
Drug |
MoA |
RoA |
Company |
Phase |
|
Ponsegromab (PF-06946860) |
GDF-15 inhibitor |
SC |
Pfizer |
IIb/III |
|
S-pindolol benzoate (ACM-001.1) |
β1 antagonist; β2 partial agonist; 5-HT1A antagonist |
Oral |
Actimed Therapeutics |
II |
|
TCMCB07 |
Melanocortin‐3/4 receptor antagonist |
SC |
Endevica Bio |
II |
|
XX |
XX |
X |
XXX |
X |
Cancer Cachexia Drug Class Analysis
Cancer cachexia is a multifactorial syndrome marked by involuntary weight loss, muscle wasting, and anorexia, commonly seen in advanced malignancies. It results from a complex interplay of systemic inflammation, metabolic disruption, and reduced intake, and significantly impacts prognosis, treatment tolerance, and quality of life.
Current pharmacologic management focuses on symptomatic improvement, with limited disease-modifying impact. Corticosteroids and progesterone analogs like megestrol acetate remain mainstays for short-term appetite stimulation and weight gain. Low-dose olanzapine is gaining attention for its appetite-promoting effects, particularly during chemotherapy. However, androgens, NSAIDs, and cannabinoids show inconsistent clinical benefits. The absence of US FDA-approved therapies underscores an ongoing need for agents that target both catabolic and inflammatory pathways.
Continued in report…
Cancer Cachexia Market Outlook
- The total market size of cancer cachexia in the 7MM was approximately USD 475 million in 2024 and is projected to increase during the forecast period (2025–2034).
- The market size of cancer cachexia in the US was approximately USD 275 million in 2024 and will increase at a CAGR of 10.9% during the forecast period driven by the increasing awareness of the disease and the launch of the emerging therapy.
- The total market size of cancer cachexia in EU4 and the UK was calculated to be approximately USD 150 million in 2024, which was nearly 32% of the total market revenue for the 7MM.
- According to DelveInsight’s estimates, among EU4 and the UK, Germany accounted for the largest market for cancer cachexia, with USD 40 million in 2024 while Spain accounted for the least with USD 22 million in 2024.
- The total market size of cancer cachexia in Japan was calculated to be USD 45 million in 2024, which is expected to increase at a CAGR of 2.6% in the forecast period (2025–2034).
- The current standard of care for cancer cachexia, which includes progestins, corticosteroids, and Tumor Necrosis Factor (TNF) inhibitors, among others provides modest clinical benefit and, as per DelveInsight estimates, generated approximately USD 470 million in 2024, highlighting a substantial unmet need in this space.
- Several major players, including Pfizer, Actimed Therapeutics, and Endevica Bio, among others, are actively advancing therapies for cancer cachexia, with assets currently in the early to late stages of clinical development.
- Estimates suggest that ponsegromab (PF-06946860) is expected to generate approximately USD 425 million in the 7MM by 2034.
Cancer Cachexia Treatment Market Outlook
Cancer cachexia, sometimes referred to as cancer-associated fatigue, is a multifactorial syndrome characterized by progressive, often irreversible, skeletal muscle loss—with or without fat loss—that cannot be reversed through conventional nutritional support. It significantly contributes to functional decline and poor clinical outcomes.
Effective management requires a comprehensive, individualized approach integrating pharmacologic, nutritional, physical, and psychosocial interventions. Invasive artificial nutrition is generally avoided due to limited benefit and potential risks, making pharmacologic options central to care. Corticosteroids offer short-term appetite stimulation, though benefits are transient and lack long-term validation. Other agents—such as cannabinoids, cyproheptadine, hydrazine sulfate, anabolic steroids, pentoxifylline, thalidomide, and ondansetron—have shown inconsistent efficacy.
High-dose progestins (HDPs) like megestrol acetate and medroxyprogesterone acetate (MPA) remain more promising, originally developed for hormone-dependent tumors and later found to improve appetite and weight. However, their effects are palliative, not disease-modifying, and are typically used as part of a broader multimodal regimen including exercise, psychosocial care, and cancer therapy.
Approved therapies are limited. ADLUMIZ (anamorelin hydrochloride) received approval from Japan’s MHLW in January 2021 for cancer cachexia but failed to gain approval in the US and EU. The EMA’s CHMP issued a negative opinion in 2017 for use in non-small cell lung cancer, later reaffirmed after re-examination. These regulatory hurdles highlight the challenges in advancing cachexia treatments globally.
Despite high prevalence and clinical burden, cachexia remains under-recognized, with delayed diagnosis, lack of reliable biomarkers, and fragmented treatment approaches. There is an urgent need for validated, multimodal protocols that integrate pharmacologic, nutritional, exercise, and psychosocial strategies.
Addressing this gap, companies like Pfizer and Actimed Therapeutics are advancing late-stage candidates such as ponsegromab (PF-06946860) and S-pindolol benzoate (ACM-001.1), among others, that aim to offer targeted, clinically validated alternatives to improve patient outcomes in cancer cachexia.
Cancer Cachexia Drugs Uptake
This section focuses on the uptake rate of potential drugs expected to be launched in the market during 2020–2034. For example, Pfizer’s ponsegromab (PF-06946860), a monoclonal antibody targeting GDF-15, is anticipated to enter the US market by 2029, with slow-medium uptake during the forecast period.
Further detailed analysis of emerging therapies drug uptake in the report…
Cancer Cachexia Pipeline Development Activities
The report provides insights into different therapeutic candidates in Phase III, Phase II, and Phase I. It also analyzes key players involved in developing targeted therapeutics.
Pipeline development activities
The report covers information on collaborations, acquisitions and mergers, licensing, and patent details for emerging therapies for cancer cachexia.
Latest KOL Views on Cancer Cachexia
To keep up with current market trends, we take KOLs and SMEs’ opinions working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts contacted for insights on cancer cachexia evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake, along with challenges related to accessibility, including Medical/scientific writers, Medical Professionals, Professors, Directors, and Others.
DelveInsight’s analysts connected with 50+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. Centers like the Duke University Medical Center, US, Indiana University School of Medicine, US, University of Rochester Medical Center, US, University Hospital Heidelberg, Germany, Hospices Civils de Lyon, France, Université Paris Saclay, France, Azienda Ospedaliero-Universitaria Careggi, Italy, Universitat de Barcelona, Spain, University of Edinburgh, Leeds, UK, Kagoshima University, Japan, and Iida Municipal Hospital, Japan, among others, were contacted. Their opinion helps understand and validate current and emerging therapy treatment patterns or cancer cachexia market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
What KOLs are saying on Spinal Cord Injury Patient Trends?
As per the KOLs from the US, cancer cachexia results from both the tumor’s metabolic demands and treatment-related effects, causing muscle loss, fatigue, and reduced functional ability. It extends beyond simple weight loss, involving systemic decline. Distinguishing cachexia from general weight loss is crucial for timely intervention and effective supportive care in oncology.
As per the KOLs from France, cancer cachexia is highly prevalent in older cancer patients, driven by factors such as poor performance status, low food intake, impaired mobility, cognitive decline, and depression, which are more common in this age group. These overlapping vulnerabilities make the elderly particularly susceptible to rapid muscle loss and functional decline, significantly worsening prognosis and survival.
As per the KOLs from Japan, cachexia arises from complex interactions between cancer-derived factors, inflammatory and immune cells within the tumor microenvironment, and systemic disruptions involving the endocrine, metabolic, and nervous systems. These signals drive tissue-level processes such as inflammation, proteolysis, autophagy, and lipolysis, leading to skeletal and cardiac muscle wasting. As a result, patients face extensive morbidities—including metabolic imbalance, immune dysfunction, and functional decline—ultimately experiencing a substantial deterioration in overall quality of life.
Cancer Cachexia Report Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT and Attribute Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst’s discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Attribute analysis analyzes multiple emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
To analyze the effectiveness of these therapies, have calculated their attributed analysis by giving them scores based on their ability to improve atrial and ventricular dimension/function and ability to regulate heart rate.
Further, the therapies’ safety is evaluated wherein the adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials, which directly affects the safety of the molecule in the upcoming trials. It sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the route of administration, order of entry and designation, probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Cancer Cachexia Market Access and Reimbursement
ADLUMIZ (anamorelin hydrochloride)
ADLUMIZ tablet 50 mg, containing anamorelin hydrochloride, is approved for the treatment of cancer cachexia associated with non-small cell lung cancer, gastric cancer, pancreatic cancer, and colorectal cancer. The recommended adult dosage is 100 mg taken orally once daily in the fasting state.
The product was approved on January 22, 2021, and included in the National Health Insurance (NHI) reimbursement list on April 21, 2021. The NHI drug price is JPY 246.40 per tablet. It is manufactured and distributed by Ono Pharmaceutical.
Further details will be provided in the report.
The report provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenarios, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Scope of the Cancer Cachexia Market Report
- The report covers a segment of key events, an executive summary, and a descriptive overview of cancer cachexia, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, disease progression, and treatment guidelines have been provided.
- Additionally, an all-inclusive account of the current and emerging therapies and the elaborative profiles of late-stage and prominent therapies will impact the current treatment landscape.
- A detailed review of the cancer cachexia market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The report provides an edge while developing business strategies by understanding trends through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help shape and drive the 7MM cancer cachexia market.
Cancer Cachexia Market Report Insights
- Patient Population
- Therapeutic Approaches
- Cancer Cachexia Pipeline Analysis
- Cancer Cachexia Market Size and Trends
- Existing and Future Market Opportunity
Cancer Cachexia Market Report Key Strengths
- 10 years Forecast
- The 7MM Coverage
- Cancer Cachexia Epidemiology Segmentation
- Key Cross Competition
- Attribute analysis
- Drugs Uptake and Key Market Forecast Assumptions
Cancer Cachexia Market Report Assessment
- Current Treatment Practices
- Unmet Needs
- Pipeline Product Profiles
- Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
Key Questions Answered in the Cancer Cachexia Report
Cancer Cachexia Market Insights
- What was the total market size of cancer cachexia, the market size of cancer cachexia by therapies, and market share (%) distribution in 2020, and what would it look like by 2034? What are the contributing factors for this growth?
- How will ponsegromab (PF-06946860) affect the treatment paradigm of cancer cachexia?
- How will ADLUMIZ compete with similar-class products and off-label therapies?
- Which drug is going to be the largest contributor by 2034?
- What are the pricing variations among different geographies for approved and marketed therapies?
- How would future opportunities affect the market dynamics and subsequent analysis of the associated trends
Cancer Cachexia Epidemiology Insights
- What are the disease risks, burdens, and unmet needs of cancer cachexia? What will be the growth opportunities across the 7MM with respect to the patient population pertaining to cancer cachexia?
- What is the historical and forecasted cancer cachexia patient pool in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan?
- Out of the countries mentioned above, which country would have the highest diagnosed prevalent cancer cachexia population during the forecast period (2025–2034)?
- What factors are contributing to the growth of cancer cachexia cases?
Current Treatment Scenario, Marketed Drugs, and Emerging Therapies
- What are the current options for the treatment of cancer cachexia? What are the current clinical and treatment guidelines for treating cancer cachexia?
- How many companies are developing therapies for the treatment of cancer cachexia?
- How many emerging therapies are in the mid-stage and late stage of development for treating cancer cachexia?
- What are the recent novel therapies, targets, mechanisms of action, and technologies developed to overcome the limitations of existing therapies?
- What is the cost burden of current treatment on the patient?
- Patient acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the accessibility issues of approved therapy in the US?
- What is the 7MM historical and forecasted market of cancer cachexia?
Reasons to Buy Cancer Cachexia Report
- The report will help develop business strategies by understanding the latest trends and changing treatment dynamics driving the cancer cachexia market.
- Insights on patient burden/disease prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- Understand the existing market opportunities in varying geographies and the growth potential over the coming years.
- The distribution of historical and current patient share is based on real-world prescription data in the US, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
- Identifying upcoming solid players in the market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the conjoint analysis section to provide visibility around leading classes.
- Highlights of Access and Reimbursement policies for cancer cachexia, barriers to accessibility of approved therapy, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing market so that the upcoming players can strengthen their development and launch strategy.