Carcinoid Tumor Market
- A carcinoid tumor is a rare type of neuroendocrine tumor that most often occurs in the gastrointestinal tract or the lungs, with an estimated incidence of fewer than 3 cases per 100,000 individuals each year.
- It is estimated that carcinoid tumors overall encompass 1% to 5% of all lung neoplasms, and atypical carcinoid tumor represents 10% of this subpopulation. Atypical carcinoid tumor is associated with higher rates of metastases and worse survival (3-year survival of 67% in all patients and 26% in those with metastatic disease).
- Approximately 60–70% of carcinoids occur in the gastrointestinal tract, and the second most common site is the tracheobronchial tree.
- Seventy-five percent (75%) of patients with carcinoid tumors develop hepatic metastases regardless of the location of the primary tumor.
- The 5-year survival rates for gastrointestinal carcinoid tumors vary by stage at diagnosis: approximately 97% for localized disease, 96% for regional spread, and 72% for distant metastases.
- Surgery is the primary treatment for carcinoid tumors. Additional therapeutic options include somatostatin analogs, targeted therapy, chemotherapy, and radiation therapy. External radiation therapy has a limited role and is generally reserved for treating metastases in the bones and brain.
- The key players in the carcinoid tumor market include Crinetics Pharmaceuticals and Sanwa Kagaku Kenkyusho (PALSONIFY [paltusotine]), RayzeBio (RYZ101), EpimAb Biotherapeutics (EMB-01), and others. These companies are developing new treatment options for carcinoid tumors and are expanding their product portfolio in this market.
Factors affecting Carcinoid Tumor Market Growth
-
Increasing Incidence of Carcinoid Tumors
The rising number of diagnosed cases, particularly gastrointestinal and pulmonary carcinoid tumors, is driving the demand for effective treatments.
-
Advancements in Diagnostic Techniques
Improved imaging technologies, biomarker identification, and endoscopic methods allow for earlier and more accurate detection, supporting better patient outcomes.
-
Development of Novel Therapies
Introduction of targeted therapies, peptide receptor radionuclide therapy (PRRT), and advanced surgical approaches is enhancing treatment efficacy and patient survival.
-
Growing Awareness Among Healthcare Professionals
Enhanced understanding of carcinoid tumor management among oncologists and endocrinologists is contributing to increased adoption of specialized treatment regimens.
-
Rising Investment in Research and Clinical Trials
Continuous investment in R&D for innovative therapies and ongoing clinical trials are expanding treatment options and driving market growth.
-
Aging Population
The global increase in elderly populations, who are more susceptible to neuroendocrine tumors, is contributing to higher demand for effective therapies.
-
Expansion of Healthcare Infrastructure
Improved access to specialized oncology and surgical centers, especially in emerging markets, supports increased treatment adoption.
DelveInsight's “Carcinoid Tumor – Market Insight, Epidemiology and Market Forecast – 2034” report delivers an in-depth analysis of Carcinoid Tumor epidemiology, market, and clinical development in Carcinoid Tumor. In addition to this, the report provides historical and forecasted epidemiology and market data as well as a detailed analysis of the Carcinoid Tumor market trends in the United States, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
The Carcinoid Tumor market report provides real-world prescription pattern analysis, emerging drugs assessment, market share, and uptake/adoption pattern of individual therapies, as well as historical and forecasted Carcinoid Tumor market size from 2020 to 2034 in 7MM. The report also covers current Carcinoid Tumor treatment practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s underlying potential.
Geography Covered
- The United States
- EU4 (Germany, France, Italy, and Spain) and the United Kingdom
- Japan
|
Study Period |
2020–2034 |
|
Forecast Period |
2025–2034 |
|
Geographies Covered |
US, EU4 (Germany, France, Italy, and Spain), the UK, and Japan |
|
Carcinoid Tumor Epidemiology |
Segmented by:
|
|
Carcinoid Tumor Key Companies |
|
|
Carcinoid Tumor Key Therapies |
|
|
Carcinoid Tumor Market |
Segmented by:
|
|
Analysis |
|
Carcinoid Tumor Disease Understanding
Carcinoid Tumor Overview
A carcinoid tumor is a rare type of neuroendocrine tumor that typically grows slowly. Carcinoid tumors can arise in several places throughout the body and are characterized by the production of an excess of hormone-like substances, such as serotonin, bradykinin, histamine, and prostaglandins, which leads to other associated complications. These tumors are a subset of neuroendocrine tumors and usually begin in the digestive tract (stomach, appendix, small intestine, colon, and rectum) or the lungs.
Types of carcinoid tumors include
- Slow-growing tumors: These tumors are the most common type. They usually remain small, under about an inch wide. They don’t grow rapidly or spread to other parts of the body.
- Faster-growing tumors: These tumors may grow more rapidly and are more likely to spread.
- Hormone-secreting (functional) tumors: These functioning carcinoid tumors produce hormones, including serotonin. The effect of serotonin and other hormones causes the symptoms known as carcinoid syndrome. The symptoms of carcinoid tumors can vary depending on the size, location, and stage of the tumor. Some of the most common symptoms include flushing, diarrhea, heart palpitations, wheezing, and shortness of breath.
- Non-hormone-secreting (nonfunctional) tumors: These carcinoid tumors are the most common. They do not make any hormones.
Carcinoid Tumor Diagnosis
Carcinoid tumor is diagnosed based on a combination of factors, including the patient's symptoms, a physical exam, and blood tests. Blood tests can measure the levels of hormones that are produced by the tumor. Imaging scans like CT, MRI, PET, X-ray, and nuclear medicine scans are used to precisely locate the tumor. Endoscopy and bronchoscopy are employed for internal visualizations. Biopsy procedures, such as needle extraction or surgical removal, provide tissue samples for laboratory analysis to confirm the tumor type and aggressiveness. Endoscopy can be used to view the tumor and to collect tissue samples for biopsy.
Further details related to country-based variations in diagnosis are provided in the report
Carcinoid Tumor Treatment
The treatment of carcinoid tumors depends on the size and location of the tumor, as well as the severity of the symptoms. Surgery is the most effective treatment for small carcinoid tumors that have not metastasized. Surgery for these tumors can cure them. Once a tumor has spread or become too big to remove, other treatments may still work well. One of the most common treatments for carcinoid syndrome is somatostatin analog therapy. Somatostatin analogs are medications that block the release of hormones from the tumor. They are typically given as injections under the skin. Other treatments for carcinoid tumors include chemotherapy, targeted therapy, radiotherapy, and peptide receptor radionuclide therapy.
Carcinoid Tumor Epidemiology
The Carcinoid Tumor cancer epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented as total incident population of carcinoid tumor, gender-specific incident population of carcinoid tumor, origin-specific incident population of carcinoid tumor, age-specific incident population of carcinoid tumor, stage-specific incident population of carcinoid tumor and total treated cases of carcinoid tumor in the 7MM, covering the United States, EU4 (Germany, France, Italy, and Spain), and the United Kingdom, and Japan from 2020 to 2034.
- Among all carcinoid tumors, those arising in the lungs account for approximately 25% of cases, making them the second most common site.
- Fewer than 10% of people with carcinoid tumors develop symptoms. The incidence of symptoms, however, may vary based on the location of the tumor. Because carcinoid tumors grow so slowly, they are usually not diagnosed until age 55 to 65.
- According to secondary research, about 12,000 people in the United States will be diagnosed with neuroendocrine tumors — sometimes called carcinoid tumors — each year.
- Approximately 40% of all gastrointestinal carcinoid tumors are located in the small intestine, while pulmonary carcinoid tumors represent about 1–2% of all lung cancers.
- Secondary research suggests that appendiceal carcinoids showed an unusually early onset with a maximum incidence at age 15–19 years for women and 20–29 years for men.
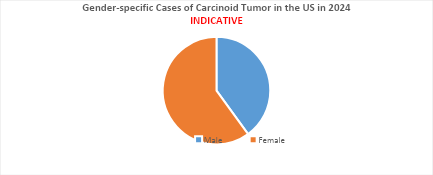
- In the US, the median age at diagnosis for carcinoid tumors is 60 years, with the condition being more common in women than in men.
Carcinoid Tumor Drug Chapters
The drug chapter segment of the Carcinoid Tumor report encloses a detailed analysis of Carcinoid Tumor’s late-stage (Phase III and Phase I/II) pipeline drugs. It also deep dives into Carcinoid Tumor’s pivotal clinical trial details, recent and expected market approvals, patent details, the latest news, and recent deals and collaborations.
Carcinoid Tumor Marketed Drugs
- SOMATULINE AUTOGEL /SOMATULINE DEPOT (lanreotide): Ipsen Biopharmaceuticals
SOMATULINE AUTOGEL /SOMATULINE DEPOT is made of the active substance lanreotide and is a long-acting somatostatin analogue that inhibits the secretion of growth hormone and certain hormones secreted by the digestive system. The new electronic auto-injector is designed to enhance the treatment experience for individuals living with carcinoid syndrome, along with gastroenteropancreatic neuroendocrine tumors and acromegaly.
In September 2017, the US Food and Drug Administration (FDA) approved a supplemental indication for SOMATULINE DEPOT for the treatment of carcinoid syndrome; when used, it reduces the frequency of short-acting somatostatin analogue rescue therapy.
- SANDOSTATIN LAR DEPOT (octreotide acetate): Novartis
SANDOSTATIN LAR DEPOT (octreotide acetate) is a somatostatin analog indicated for patients with metastatic carcinoid tumors who experience severe diarrhea and flushing, and who have previously responded to and tolerated subcutaneous Sandostatin injections. Additionally, it is also approved for long-term maintenance therapy in acromegalic patients who have had an inadequate response to surgery and/or radiotherapy, or for whom surgery and/or radiotherapy is not an option, and long-term treatment of the profuse watery diarrhea associated with VIP-secreting tumors.
In October 2024, Teva Pharmaceuticals launched the first and only generic version of SANDOSTATIN LAR DEPOT in the United States.
|
Table 1: Key Cross of Marketed Drug | ||||||
|
Drug |
Company |
Indication |
Molecule Type |
MoA |
RoA |
Marketed Region |
|
SOMATULINE DEPOT (lanreotide) |
Ipsen |
Adults with carcinoid syndrome |
Synthetic octapeptide |
SST2 agonist |
SC |
US: 2017 |
|
SANDOSTATIN LAR DEPOT (octreotide acetate) |
Novartis |
Severe diarrhea/flushing episodes associated with metastatic carcinoid tumors |
Acetate salt of a cyclic octapeptide |
SST2 agonist |
Intramuscular |
US: 1998 EU: 2014 |
|
XERMELO (telotristat ethyl) |
Lexicon Pharmaceuticals |
Carcinoid syndrome diarrhea |
Small molecule |
Tryptophan hydroxylase (TPH) inhibitor |
Oral |
US: 2017 EU: 2017 |
Carcinoid Tumor Emerging Drugs
- PALSONIFY (paltusotine): Crinetics Pharmaceuticals and Sanwa Kagaku Kenkyusho
Paltusotine is the first oral, once-daily, selectively-targeted SST2 agonist and is currently in Phase III clinical development for carcinoid syndrome associated with neuroendocrine tumors. Additionally, it is also in investigational Phase III studies for acromegaly. Phase II clinical trials (NCT07087054) began in August 2025 for patients with carcinoid syndrome caused by well-differentiated neuroendocrine tumors.
In March 2024, Crinetics Pharmaceuticals announced positive topline results from its Phase II (NCT05361668) study of oral paltusotine in carcinoid syndrome. The drug showed rapid, sustained symptom relief—reducing flushing by 63%, excess bowel movements by 60%, flushing severity by 61%, and urgency by 64%. Benefits were seen within 2 weeks and lasted 8 weeks. Paltusotine was well-tolerated, with no serious treatment-related adverse events. Biomarker data supported its activity.
- RYZ101: RayzeBio
RYZ101 is an investigational targeted radiopharmaceutical therapy, designed to deliver a highly potent radioisotope, Actinium-225 (Ac225), to tumors expressing SSTR2. RYZ101 is being evaluated in clinical studies for patients with SSTR+ GEP-NETs, including carcinoid tumors and newly diagnosed extensive-stage small-cell lung cancer.
The drug is currently undergoing evaluation in a Phase III clinical trial.
|
Comparison of Emerging Drugs Under Development | ||||||
|
Drug Name |
Company |
Highest Phase |
Indication |
RoA |
MoA |
Molecule Type |
|
Paltusotine |
Crinetics Pharmaceuticals and Sanwa Kagaku Kenkyusho |
III |
Carcinoid syndrome due to well-differentiated neuroendocrine tumors |
Oral |
SST2 agonist |
Small molecule |
|
RYZ101 |
RayzeBio |
III |
Carcinoid tumor |
IV infusion |
Alpha-emitting radiopharmaceutical agent |
Radiopharmaceutical therapy |
|
[212Pb] VMT-α-NET |
Perspective Therapeutics |
I/II |
Neuroendocrine tumor carcinoid, and carcinoid tumor of the GI system |
IV infusion |
SSTR2 targeting peptide |
Radioligand therapy |
|
EMB-01 |
EpimAb Biotherapeutics |
I/II |
Metastatic gastrointestinal carcinoid tumor |
IV infusion |
EGFR/cMET bispecific antibody |
Bispecific antibody |
Note: Detailed emerging therapies assessment will be provided in the final report.
Carcinoid Tumor Drug Class Insights
Somatostatin Analogs
Somatostatin analogs are a class of drugs that work by blocking the release of hormones from carcinoid tumors. Somatostatin analogs are the most commonly used drug class for carcinoid tumors. They are effective in controlling symptoms, such as flushing, diarrhea, and wheezing.
Chemotherapy Drugs
Chemotherapy drugs kill cancer cells by damaging their DNA. Chemotherapy drugs are often used to treat carcinoid tumors that have spread to other parts of the body. They can be used alone or in combination with other treatments.
Targeted Therapy
These drugs target specific molecules involved in cancer growth and spread. Targeted therapy drugs are a newer class of drugs that are specifically designed to target molecules involved in cancer growth and spread. They are often used to treat carcinoid tumors that have not responded to other treatments.
Carcinoid Tumor Market Outlook
The current treatment approach for carcinoid tumors focuses primarily on controlling symptoms and stabilizing tumor growth through well-established, evidence-based therapies. Somatostatin analogs, including octreotide and lanreotide, serve as the foundation of treatment by effectively suppressing hormone-related symptoms and inhibiting tumor progression. In cases where symptoms persist despite somatostatin analog therapy, telotristat ethyl is introduced to manage refractory diarrhea by blocking serotonin synthesis. Surgical resection continues to play a key role in eligible patients, while liver-directed treatments like embolization are commonly used for those with liver metastases. Although chemotherapy and interferon-alpha are occasionally considered, their overall effectiveness is limited and often accompanied by substantial side effects.
SANDOSTATIN LAR (octreotide acetate for injectable suspension) was the first SSA approved in the US in 1998 to treat patients with certain symptoms associated with carcinoid tumors and other types of functional gastrointestinal and pancreatic neuroendocrine tumors.
The future of carcinoid tumor treatment is moving toward more personalized and targeted therapies. Leading companies like Crinetics Pharmaceuticals, Sanwa Kagaku Kenkyusho, RayzeBio, EpimAb Biotherapeutics, and others are advancing their lead candidates through various stages of clinical development for the treatment of carcinoid tumors.
Carcinoid Tumor drug uptake
This section focuses on the uptake rate of potential drugs expected to be launched in the market during 2025–2034, which depends on the competitive landscape, safety, and efficacy data, along with the order of entry.
Further detailed analysis of emerging therapies' drug uptake in the report…
Carcinoid Tumor Pipeline Development Activities
The report provides insights into different therapeutic candidates in (Phase III and Phase I/II). It also analyzes key players involved in developing targeted therapeutics.
Pipeline Development Activities
The report covers information on collaborations, acquisitions and mergers, licensing, and patent details for Carcinoid Tumor cancer therapies.
Latest KOL Views on the Carcinoid Tumor Market
To keep up with the real-world scenario in current and emerging market trends, we take opinions from Key Industry Leaders working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts were contacted for insights on the evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake, along with challenges related to accessibility, including MDs, Medical/scientific writers, Professors, and Others.
DelveInsight’s analysts connected with 20+ KOLs to gather insights; however, interviews were conducted with 10+ KOLs in the 7MM. Centers such as Louisiana State University Health Science Center-Shreveport, etc., were contacted. Their opinion helps understand and validate current and emerging therapy treatment patterns or Carcinoid Tumor market trends.
|
KOL Views |
|
“Carcinoid crisis is a potentially life-threatening complication characterized by severe hemodynamic instability, flushing, bronchoconstriction, and hypotension, often triggered by surgery, anesthesia, or tumor necrosis. It typically occurs in patients with high levels of circulating vasoactive peptides, e.g., serotonin. Prophylactic administration of octreotide is recommended in patients not on somatostatin analogs as part of treatment.” MD, Louisiana State University Health Science Center-Shreveport, US |
Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT Analysis and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of gaps in disease diagnosis, patient awareness, physician acceptability, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided.
Conjoint Analysis analyzes multiple approved and emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
In efficacy, the trial’s primary and secondary outcome measures are evaluated; for instance, one of the most important primary outcome measures is event-free survival and overall survival.
Further, the therapies’ safety is evaluated, wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the probability of success and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Carcinoid Tumor Market Access and Reimbursement
Reimbursement may be referred to as the negotiation of a price between a manufacturer and a payer that allows the manufacturer access to the market. It is provided to reduce the high costs and make the essential drugs affordable. Health technology assessment (HTA) plays an important role in reimbursement decision-making and recommending the use of a drug. These recommendations vary widely throughout the seven major markets, even for the same drug. In the US healthcare system, both Public and Private health insurance coverage are included. Also, Medicare and Medicaid are the largest government-funded programs in the US. The major healthcare programs, including Medicare, Medicaid, Health Insurance Program (CHIP), and the state and federal health insurance marketplaces, are overseen by the Centers for Medicare & Medicaid Services (CMS). Other than these, Pharmacy Benefit Managers (PBMs) and third-party organizations that provide services and educational programs to aid patients are also present.
The report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of currently used therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Scope of the Carcinoid Tumor Report
- The report covers a segment of key events, an executive summary, a descriptive overview of Carcinoid Tumor, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight has been provided into the epidemiology segments and forecasts, the future growth potential of the diagnosis rate, and disease progression along treatment guidelines.
- Additionally, an all-inclusive account of both the current and emerging therapies, along with the elaborative profiles of late-stage and prominent therapies, will have an impact on the current treatment landscape.
- A detailed review of the Carcinoid Tumor market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help in shaping and driving the 7MM Carcinoid Tumor market.
Carcinoid Tumor Report Insights
- Patient Population
- Therapeutic Approaches
- Carcinoid Tumor Pipeline Analysis
- Carcinoid Tumor Market Size and Trends
- Existing and Future Market Opportunity
Carcinoid Tumor Report Key Strengths
- Ten-Years Forecast
- 7MM Coverage
- Carcinoid Tumor Epidemiology Segmentation
- Key Cross Competition
- Conjoint analysis
- Drugs Uptake and Key Market Forecast Assumptions
Carcinoid Tumor Report Assessment
- Current Treatment Practices
- Unmet Needs
- Pipeline Product Profiles
- Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
FAQs
- What is the historical and forecasted Carcinoid Tumor patient pool/patient burden in the United States, EU4 (Germany, France, Italy, and Spain), and the United Kingdom, and Japan?
- How would the market drivers, barriers, and future opportunities affect the market dynamics and subsequent analysis of the associated trends?
- What are the current and emerging options for the treatment of Carcinoid Tumor?
- What are the recent novel therapies, targets, mechanisms of action, and technologies developed to overcome the limitations of existing therapies?
- Patient acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the country-specific accessibility issues of expensive, recently approved therapies?
- How many key players are developing therapies for Carcinoid Tumor?
- Which drug is the major contributor to the Carcinoid Tumor market by 2034?
Reasons to buy the Carcinoid Tumor Market Report
- The report will help in developing business strategies by understanding the latest trends and changing treatment dynamics driving the Carcinoid Tumor market.
- Insights on patient burden/disease Incidence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- Understand the existing market opportunities in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identifying strong upcoming players in the market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of class-wise potential emerging therapies under the conjoint analysis section to provide visibility around leading classes.
- Highlights of access and reimbursement policies of approved therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing market so that the upcoming players can strengthen their development and launch strategy.





