Catheter-Related Bloodstream Infection Market
- Citius Pharmaceuticals licensed the worldwide rights to MINO-LOK from the University of Texas MD Anderson Cancer Center.
- MINO-LOK was granted Qualified infectious disease product (QIDP) and Fast Track designation (FTD) by the FDA and has patent protection through 2024 and formulation patent protection through 2036.
- In November 2023, DEFENCATH by CorMedix got FDA approval for the treatment of Catheter-related bloodstream infections (CRBSI) in adult patients with kidney failure receiving chronic hemodialysis (HD) through a central venous catheter. Securing approval in Europe and Japan could further expand its market size.
- In May 2024, Citius Pharmaceuticals announced positive topline results of its pivotal Phase III clinical trial of Mino-Lok. The study met its primary endpoint with a statistically significant improvement in the time to failure event in patients receiving Mino-Lok compared to control arm patients receiving clinician-directed anti-infective lock solution.
- CRBSI Incidence, speed of emergence of CRBSIs and associated clinical burden is not well-established.
- The Catheter Related Bloodstream Infection Pipeline is not so robust. Citius Pharmaceuticals is the only one developing a drug to treat catheter-related bloodstream infections.
- An increase in the Catheter Related Bloodstream Infection Incident Cases and the expected launch of emerging therapies will boost the market in the forecasted period.
Request for unlocking the sample page of the "Catheter Related Bloodstream Infection Treatment Market"
DelveInsight's “Catheter-related Bloodstream Infection Market Insight, Epidemiology and Market Forecast – 2034” report delivers an in-depth analysis of catheter-related bloodstream infection epidemiology, market, and clinical development in catheter-related bloodstream infection. In addition to this, the Catheter Related Bloodstream Infection Treatment Market Report provides historical and forecasted epidemiology and market data as well as a detailed analysis of the catheter-related bloodstream infection market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
Catheter Related Bloodstream Infection Treatment Market report provides real-world prescription pattern analysis, emerging drugs assessment, market share, and uptake/adoption pattern of individual therapies, as well as historical and forecasted catheter-related bloodstream infection market size from 2020 to 2034 in 7MM. The Catheter Related Bloodstream Infection Drugs Market Report also covers current catheter-related bloodstream infection treatment market practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s underlying potential.
|
Study Period |
2020–2034 |
|
Forecast Period |
2024–2034 |
|
Geographies Covered |
US, EU4 (Germany, France, Italy, and Spain) and the UK, and Japan |
|
Catheter-related bloodstream infection Epidemiology
|
Segmented by
|
|
Catheter-related bloodstream infection Companies |
|
|
Catheter-related bloodstream infection key therapy |
|
|
Catheter-related bloodstream infection Market |
Segmented by:
|
|
Analysis |
|
Catheter-related bloodstream infection Treatment Market: Understanding and Algorithm
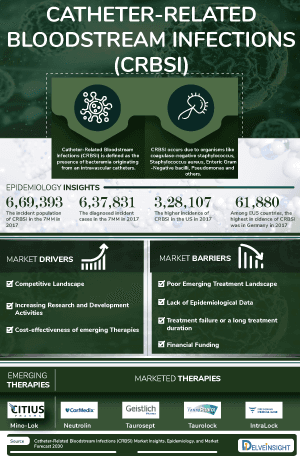
Catheter-related bloodstream infection (CRBSI, also called catheter-related sepsis) is defined as the presence of bacteremia originating from an intravenous catheter. It is one of the most frequent, lethal, and costly complications of central venous catheterization. It is also the most common cause of nosocomial bacteremia. Catheter-related bloodstream infection occurs due to organisms like coagulase-negative staphylococcus, Staphylococcus aureus, enteric gram-negative bacilli, enterococci and streptococci, pseudomonas, and others.
The diagnosis of catheter-related bloodstream infection is often suspected clinically in a patient using a central venous catheterization who presents with fever or chills, unexplained hypotension, and no other localizing sign. Mild symptoms include malaise and nausea, and severe symptoms include high fever with rigors, hypotension, vomiting, and changes in mental status in the setting of a normal catheter exit site or tunnel, on physical examination.
The catheter-related bloodstream infection report provides an overview of catheter-related bloodstream infection pathophysiology, diagnostic approaches, and detailed treatment algorithm along with a real-world scenario of a patient’s journey beginning from the first symptom, the time taken for diagnosis to the entire treatment process.
Catheter-related bloodstream infection Treatment
A catheter-associated bloodstream infection is serious but often can be successfully treated with antibiotics. The catheter might need to be removed if one develops an infection. Different measures are used to reduce the risk of occurrence of Catheter-related bloodstream infection which includes the use of utmost barrier, precautions during catheter insertion, effective cutaneous anti-sepsis, and preventive strategies based on inhibiting micro-organisms originating from the skin or catheter hub from adhering to the catheter.
Catheter-related bloodstream infection Epidemiology
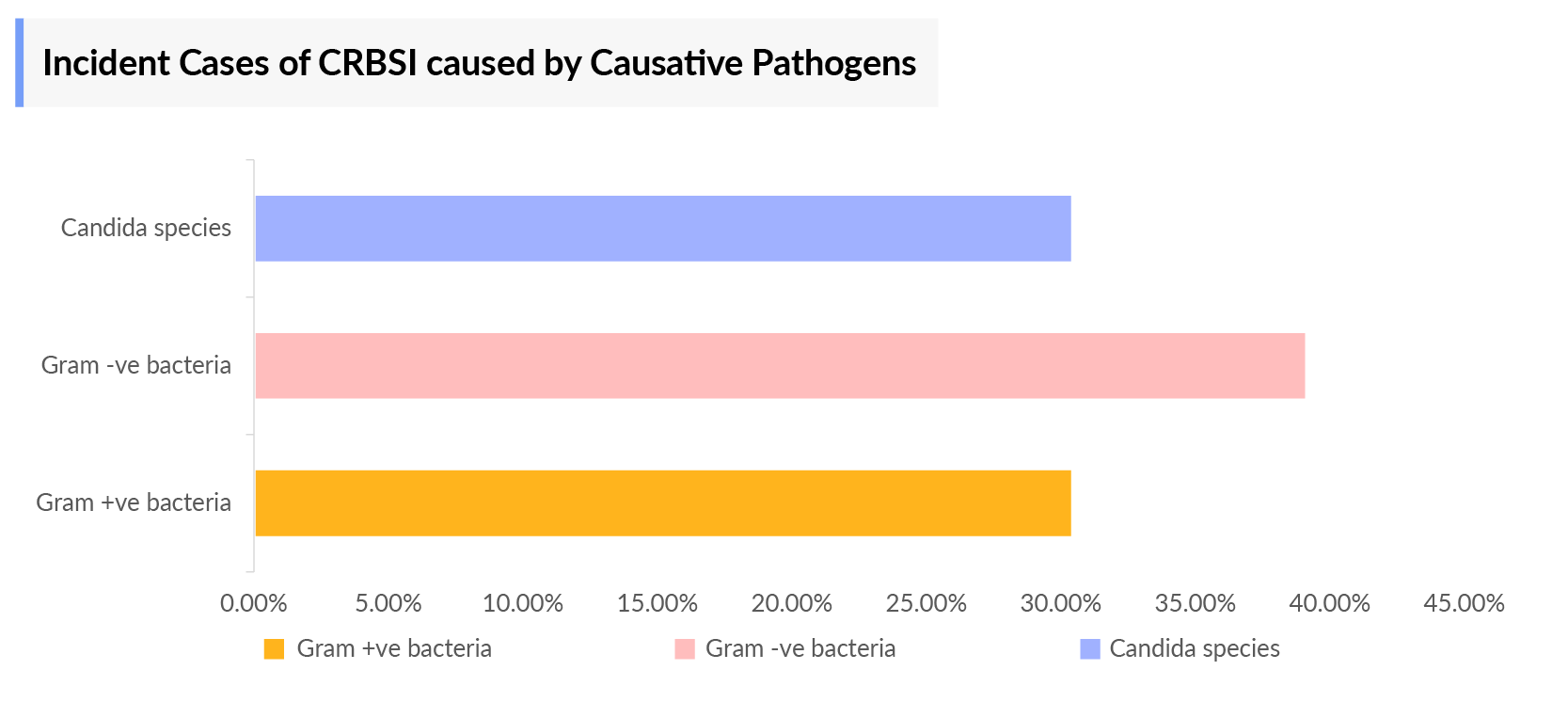
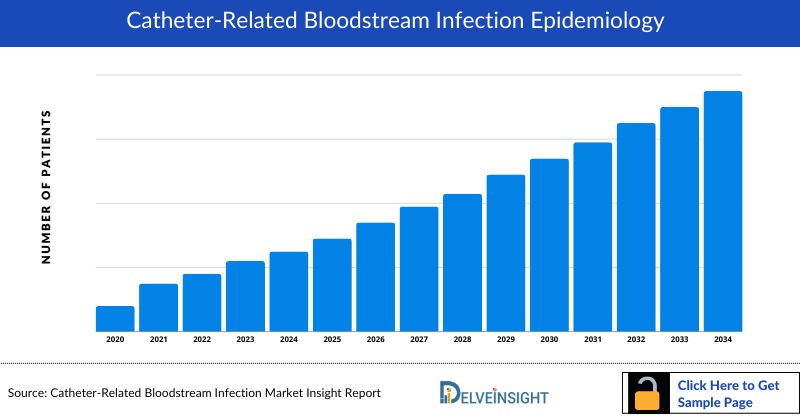
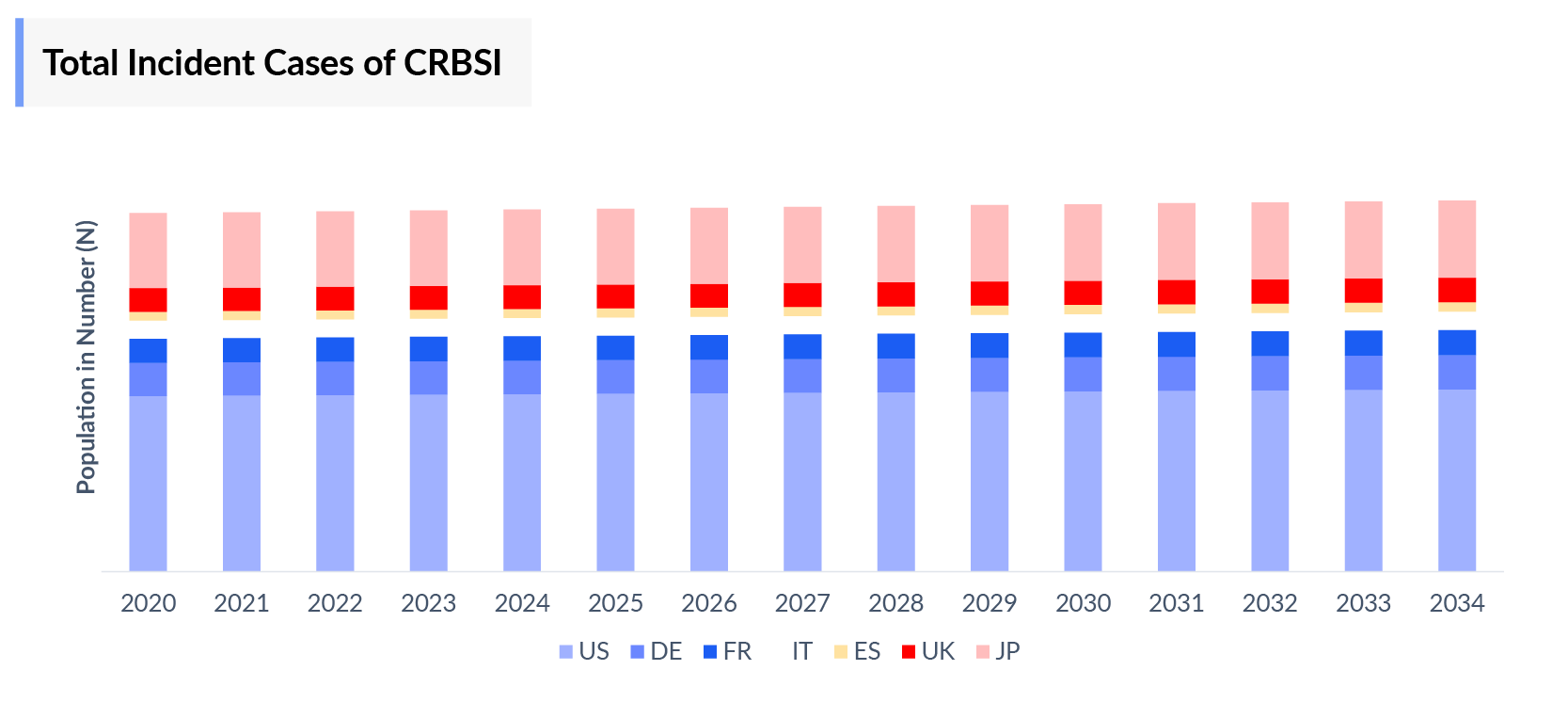
The Catheter-related bloodstream infection epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented as Total Incident Cases of Catheter-related bloodstream infection, Total Diagnosed Incident Cases of Catheter-related bloodstream infection, and Diagnosed Incident Cases by Causative pathogens of Catheter-related bloodstream infection in the 7MM covering the United States, EU4 (Germany, France, Italy, and Spain), and United Kingdom, and Japan from 2020 to 2034.
- Among 7MM, the United States accounted for the highest Catheter Related Bloodstream Infection Incident cases in 2023.
- As per the analysis, in the US, the incidence of Gram-positive bacteria is more than Gram-negative bacteria and very few are affected by candida species (fungi). Methicillin-susceptible Staphylococcus aureus occupies the maximum patient pool among the Gram-positive bacteria.
- Amongst EU4 and the UK, Germany had the highest number of Incident cases of Catheter-related bloodstream infection. On the other hand, Spain had the lowest Incident cases in 2023.
Unlock comprehensive insights! Click Here to Purchase the Full Epidemiology Report @ Catheter Related Bloodstream Infection Prevalence
Catheter-related bloodstream infection Drug Chapters
The drug chapter segment of the catheter-related bloodstream infection treatment market report encloses a detailed analysis of catheter-related bloodstream infection marketed drugs and late-stage (Phase III and Phase II) Catheter Related Bloodstream Infection pipeline drugs. It also deep dives into the catheter-related bloodstream infection pivotal clinical trial details, recent and expected market approvals, patent details, the latest news, and recent deals and collaborations.
Catheter Related Bloodstream Infection Marketed Drugs
- DEFENCATH: CorMedix
DEFENCATH is a combination of taurolidine, a thiadiazinane antimicrobial, and heparin, an anti-coagulant, indicated to reduce the incidence of catheter-related bloodstream infections in adult patients with kidney failure receiving chronic hemodialysis through a central venous catheter. This drug is indicated for use in a limited and specific population of patients. In November 2023, the US Food and Drug Administration approved DEFENCATH, a combination catheter. DEFENCATH works by leveraging taurolidine's ability to kill bacteria and combining it with heparin's blood-thinning properties to prevent catheter blockages, thus reducing the heightened risk in this group of patients.
Note: Detailed current therapy assessment will be provided in the full report of Catheter-related bloodstream infection
Catheter Related Bloodstream Infection Emerging Drugs
- MINO-LOK: Citius Pharmaceuticals
MINO-LOK is an antibiotic lock solution used to treat patients with catheter-related bloodstream infections and central line-associated bloodstream infections (CLABSIs), MINO-LOK is the only therapy under investigation to salvage infected central venous catheterization. In a Phase IIb trial, MINO-LOK demonstrated a 100% efficacy rate in salvaging colonized central venous catheterization; MINO-LOK had no significant adverse events compared to an 18% serious adverse event rate when infected central venous catheterization was removed and replaced. A multicenter Phase III pivotal superiority trial is currently underway. MINO-LOK contains a proprietary combination of minocycline, edetate (disodium EDTA), and ethyl alcohol, all of which act synergistically to break down bacterial biofilms, eradicate the bacteria, provide anti-clotting properties to maintain patency in central venous catheterization and salvage the indwelling catheter. MINO-LOK is used in two-hour locking cycles, allowing central venous catheterization to be used for its intended purposes for the remaining 22 hours each day.
Catheter-related bloodstream infection Market Outlook
If the infection is suspected, the first thing suggested by the various guidelines is to remove the catheter. If the patient remains febrile, then antibiotics and antifungal treatments are suggested. Antibiotic therapy is often empirically initiated. Among the class of antibacterial, daptomycin, vancomycin, cefazolin, ampicillin, ciprofloxacin, amikacin, and teicoplanin are several majorly prescribed off-label treatment for Catheter-related bloodstream infection management and antifungals like Fluconazole and Amphotericin B are indicated as an off-label treatment for the management of catheter-related bloodstream infection. The therapeutic landscape for Catheter-related bloodstream infection management is attributed to change, however at a moderate rate because only one pharmaceutical company is in the late-stage development of a novel solution for Catheter-related bloodstream infection.
Key Catheter Related Bloodstream Infection Companies, such as Citius Pharmaceuticals are evaluating their lead candidates in different stages of clinical development, respectively. They aim to investigate their products for the treatment of Catheter-related bloodstream infection.
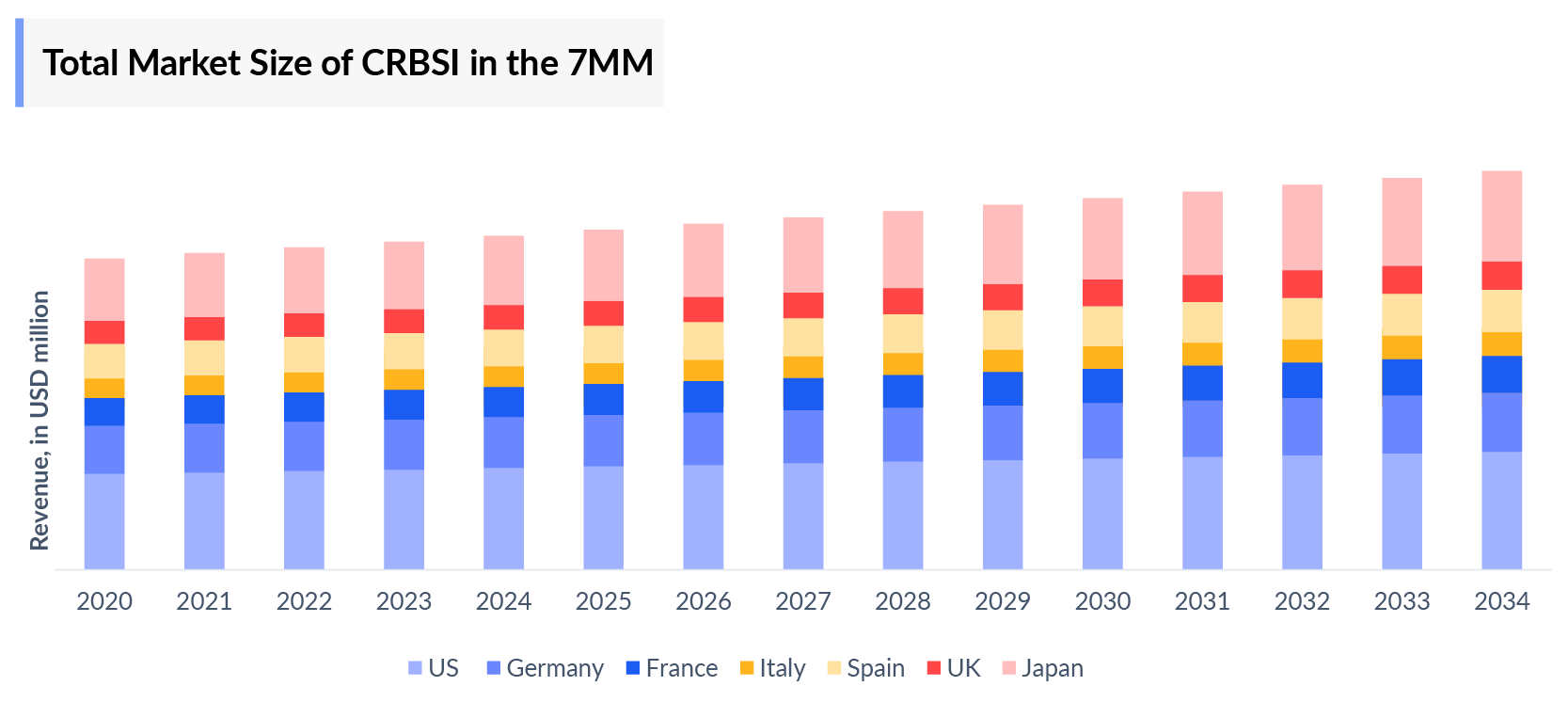
- DEFENCATH, approved in the US for the treatment of catheter-related bloodstream infections, has the potential to significantly expand its market size with anticipated approvals in Europe and Japan. Increased patient awareness, updated diagnosis and treatment guidelines, and a growing population affected by the disease are expected to drive this growth during the forecast period.
- Among 7MM, the United States accounts for the highest market size of catheter-related bloodstream infection in 2023.
Catheter-related bloodstream infection Drug Uptake
The impressive Phase IIb trial results of MINO-LOK showing a 100% efficacy rate in salvaging colonized central venous catheters and minimal adverse events compared to the alternative of catheter removal and replacement with an 18% serious adverse event rate, MINO-LOK stands out as a promising solution. As the sole drug in the pipeline addressing this critical need, it has garnered significant attention for its potential to revolutionize catheter-related infection management.
This section focuses on the uptake rate of potential Catheter Related Bloodstream Infection drugs expected to be launched in the market during 2020–2034, which depends on the competitive landscape, safety, and efficacy data along with order of entry. It is important to understand that the key Catheter Related Bloodstream Infection Companies evaluating their novel therapies in the pivotal and confirmatory trials should remain vigilant when selecting appropriate comparators to stand the greatest chance of a positive opinion from regulatory bodies, leading to approval, smooth launch, and rapid uptake.
Further detailed analysis of emerging therapy drug uptake in the report…
Catheter-related bloodstream infection Cancer Pipeline Development Activities
The Catheter Related Bloodstream Infection therapeutics market report provides insights into different therapeutic candidates in Phase III and Phase II stages. It also analyzes key Catheter Related Bloodstream Infection Companies involved in developing targeted therapeutics.
Pipeline Development Activities
The Catheter Related Bloodstream Infection therapeutics market report covers information on collaborations, acquisitions and mergers, licensing, and patent details for Catheter-related bloodstream infection therapies.
Take Your Research to the Next Level! Click Here to Get Access to the Full Pipeline Report @ Catheter Related Bloodstream Infection Treatment Drugs
KOL Views
To keep up with the real-world scenario in current and emerging market trends, we take opinions from Key Industry leaders working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts contacted for insights on the evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake along with challenges related to accessibility, including Medical/scientific writers, Doctors, Professors, and Others.
DelveInsight’s analysts connected with 20+ KOLs to gather insights; however, interviews were conducted with 10+ KOLs in the 7MM. Centers such as Cambridge University, MD Anderson Cancer Center etc., were contacted. Their opinion helps understand and validate current and emerging therapy treatment patterns or Catheter-related bloodstream infection market trends.
|
KOL Views |
|
“Almost 100% of patients admitted in ICU are subject to catheter, most of them have more than one venous catheter and arterial catheter.” |
|
“In the Intensive Care Unit (ICU), most patients have central venous catheterization and these are often replaced to rule out sepsis. However, catheter days are higher in ICU.” |
Qualitative Analysis
We perform Qualitative and Catheter Related Bloodstream Infection Therapeutics Market Intelligence analysis using various approaches, such as SWOT analysis and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of gaps in disease diagnosis, patient awareness, physician acceptability, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided.
Conjoint Analysis analyzes multiple approved and emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy. In efficacy, the trial’s primary and secondary outcome measures are evaluated; for instance, in event-free survival, one of the most important primary outcome measures is event-free survival and overall survival.
Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Catheter Related Bloodstream Infection Therapeutics Market Access and Reimbursement
Reimbursement may be referred to as the negotiation of a price between a manufacturer and payer that allows the manufacturer access to the market. It is provided to reduce the high costs and make the essential drugs affordable. Health technology assessment (HTA) plays an important role in reimbursement decision-making and recommending the use of a drug. These recommendations vary widely throughout the seven major markets, even for the same drug. In the US healthcare system, both Public and Private health insurance coverage are included. Also, Medicare and Medicaid are the largest government-funded programs in the US. The major healthcare programs including Medicare, Medicaid, Health Insurance Program (CHIP), and the state and federal health insurance marketplaces are overseen by the Centers for Medicare & Medicaid Services (CMS). Other than these, Pharmacy Benefit Managers (PBMs), and third-party organizations that provide services, and educational programs to aid patients are also present.
The Catheter Related Bloodstream Infection therapeutics market report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of currently used therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Catheter Related Bloodstream Infection Treatment Market Report Scope
- The Catheter Related Bloodstream Infection treatment market report covers a segment of key events, an executive summary, descriptive overview, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight has been provided into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, and disease progression along treatment guidelines.
- Additionally, an all-inclusive account of both the current and emerging therapies, along with the elaborative profiles of late-stage and prominent therapies, will have an impact on the current treatment landscape.
- A detailed review of the catheter-related bloodstream infection market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The Catheter Related Bloodstream Infection treatment market report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help in shaping and driving the 7MM Catheter-related bloodstream infection drugs market.
Catheter-related bloodstream infection Treatment Market Report Insights
- Patient-based Catheter Related Bloodstream Infection Market Forecasting
- Therapeutic Approaches
- Catheter-related bloodstream infection Pipeline Analysis
- Catheter-related bloodstream infection Market Size and Trends
- Existing and future Catheter Related Bloodstream Infection Drugs Market Opportunity
Catheter-related bloodstream infection Treatment Market Report Key Strengths
- 11 Years Catheter Related Bloodstream Infection Market Forecast
- 7MM Coverage
- Catheter-related bloodstream infection Epidemiology Segmentation
- Key Cross Competition
- Conjoint analysis
- Catheter Related Bloodstream Infection Drugs Uptake
- Key Catheter Related Bloodstream Infection Market Forecast Assumptions
Catheter-related bloodstream infection Treatment Market Report Assessment
- Current Catheter Related Bloodstream Infection Treatment Market Practices
- Catheter Related Bloodstream Infection Unmet Needs
- Catheter Related Bloodstream Infection Pipeline Product Profiles
- Catheter Related Bloodstream Infection Therapeutics Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
FAQs
- What is the historical and forecasted Catheter-related bloodstream infection patient pool/patient burden in the United States, EU4 (Germany, France, Italy, and Spain), and the United Kingdom, and Japan?
- What was the total catheter-related bloodstream infection treatment market size, the Catheter Related Bloodstream Infection market size by therapies, market share (%) distribution in 2023, and what would it look like in 2034? What are the contributing factors/key catalysts for this growth?
- Which combination treatment approaches will have a significant impact on catheter-related bloodstream infection treatment market size?
- What are the pricing variations among different geographies for approved therapy?
- What are the current and emerging options for the treatment of Catheter-related bloodstream infection?
- How many companies are developing therapies for the treatment of Catheter-related bloodstream infection?
- What are the recent novel therapies, targets, mechanisms of action, and technologies developed to overcome the limitations of existing therapies?
- Patient acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the country-specific accessibility issues of expensive, recently approved therapies?
Reasons to buy
- The Catheter Related Bloodstream Infection treatment market report will help in developing business strategies by understanding the latest trends and changing treatment dynamics driving the Catheter-related bloodstream infection Drugs Market.
- Insights on patient burden/disease Catheter Related Bloodstream Infection Incidence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- Understand the existing Catheter Related Bloodstream Infection Drugs Market opportunities in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Patient-based forecast model which uses bottom-up forecasting techniques is accepted as a gold standard in pharma forecasting.
- Identifying strong upcoming players in the Catheter Related Bloodstream Infection Drugs Market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the conjoint analysis section to provide visibility around leading classes.
- Highlights of access and reimbursement policies of approved therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing market so that the upcoming players can strengthen their development and launch strategy.
Stay Updated with us for Recent Artilces
-market.png&w=256&q=75)
-pipeline.png&w=256&q=75)