Cervical Dysplasia Market
- Cervical dysplasia, also known as cervical intraepithelial neoplasia (CIN), is a precancerous condition characterized by abnormal cell growth on the cervix. It is often detected through routine Pap smears or HPV testing.
- The primary cause of cervical dysplasia is infection with high-risk strains of human papillomavirus (HPV). Persistent infection can lead to cellular changes in the cervix, progressing from mild dysplasia to severe dysplasia and potentially to cervical cancer if left untreated.
- Among the 7MM, the United States accounts for the highest number of incident cases of Cervical Dysplasia (approximately 52%).
- Among severity-specific cases, around 90% cases suffer from CIN1 in the United States.
- Treatment of cervical dysplasia depends on the severity of the condition. Mild dysplasia might not be immediately treated since it can resolve without treatment. Cervical dysplasia (CIN) management primarily depends on surgical treatment since there are no approved, disease-modifying therapies available, highlighting a significant need for non-surgical alternatives.
- In order to fulfil the unmet need, key pharmaceutical companies like Frantz Viral Therapeutics and others are working to improve the treatment landscape of Cervical Dysplasia.
- Surgery holds the leading position in market size among therapies for cervical dysplasia.
- There is a growing demand for diagnostic and treatment options. Key factors shaping the market include rising incidence rates of cervical dysplasia, primarily due to persistent human papillomavirus (HPV) infection.
- In 2023, the largest market share of Cervical Dysplasia was occupied by the United States.
DelveInsight's “Cervical Dysplasia – Market Insight, Epidemiology and Market Forecast – 2034” report delivers an in-depth analysis of Cervical Dysplasia epidemiology, market, and clinical development understanding of top oncogenic drivers/biomarkers in Cervical Dysplasia (such as HPV DNA, HPV Viral load, HPV mRNA), Addition to this report provides historical and forecasted epidemiology and market data as well as a detailed analysis on the Cervical Dysplasia market trends in the United States, EU4 (Germany, Spain, Italy, and France) and the United Kingdom, and Japan.
Cervical Dysplasia market report provides real-world prescription pattern analysis, emerging drugs assessment, market share, and uptake/adoption pattern of individual therapies, as well as historical and forecasted Cervical Dysplasia market size from 2020 to 2034 in the 7MM. The report also covers current Cervical Dysplasia treatment practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s underlying potential.
Geography Covered
The United States
EU4 (Germany, France, Italy, and Spain) and the United Kingdom
Japan
|
Study Period |
2020–2034 |
|
Forecast Period |
2024–2034 |
|
Geographies Covered |
US, EU4 (Germany, France, Italy, and Spain) and the UK, and Japan |
|
Cervical Dysplasia Epidemiology
|
Segmented by: Total Diagnosed Incident Cases of Cervical Dysplasia Total Diagnosed Incident Cases for Cervical Dysplasia by Severity (without year-on-year impact of vaccination) Total Age group-specific Cases of Cervical Dysplasia (CIN 2+, with the impact of HPV vaccination) Total Treated Cases of Cervical Dysplasia |
|
Cervical Dysplasia key companies |
Frantz Viral Therapeutics
|
|
Cervical Dysplasia treatments |
Cryosurgery, laser therapy, loop electrosurgical excision procedure (LEEP) and others |
|
Cervical Dysplasia Market |
Segmented by: · Region · Therapies |
|
Analysis |
· KOL Views · SWOT Analysis · Reimbursement · Conjoint Analysis · Unmet needs |
Cervical Dysplasia Understanding and Treatment Algorithm
Cervical Dysplasia Overview and Diagnosis
Cervical Dysplasia, also known as cervical intraepithelial neoplasia (CIN), is the abnormal growth of cells on the surface of the cervix or endocervical canal. It is caused by a common virus called human papillomavirus (HPV). It can be mild, moderate, or severe, depending on the abnormality of the cell and how much the cervical tissue is affected. Cervical dysplasia is not cancer but can become cancer and spread to nearby normal tissue. There are different types of dysplasia: mild dysplasia (CIN-1), called low-grade intraepithelial lesion (LSIL), and moderate (CIN-2) or severe (CIN-3) dysplasia, called high-grade intraepithelial lesion (HSIL).
Cervical dysplasia usually causes no symptoms and is most often discovered by a routine Pap test. The most common symptom is abnormal bleeding that starts and stops between regular menstrual periods or that occurs after sexual intercourse, douching, or a pelvic exam. Cervical cancer screening includes cytology and HPV testing, alone or in combination. Conventional cytology (a Pap test sample affixed to a slide at the time of testing) and liquid-based cytology (a newer method for collecting, transporting, and preparing cells collected by the Pap test in a liquid medium) provide comparable results.
The Cervical Dysplasia report provides an overview of Cervical Dysplasia pathophysiology, diagnostic approaches, and detailed treatment algorithm along with a real-world scenario of a patient’s journey beginning from the first symptom, the time taken for diagnosis to the entire treatment process.
The Cervical Dysplasia report provide overview of Cervical Dysplasia pathophysiology, diagnostic approaches and detailed treatment algorithm along with real-world scenario of a patient’s journey beginning from the first symptom, the time taken for diagnosis to the entire treatment process.
Further details related to country-based variations in diagnosis are provided in the report
Cervical Dysplasia Treatment
Treatment of cervical dysplasia depends on the severity of the condition. Mild dysplasia might not be immediately treated since it can resolve without treatment. Repeat Pap smears may be done every three to six months. For CIN 2 or 3, treatment can include:
- Cryotherapy
- Laser Treatment
- Conization
- Loop electrosurgical excision procedure (LEEP)
- Hysterectomy
Cervical Dysplasia Epidemiology
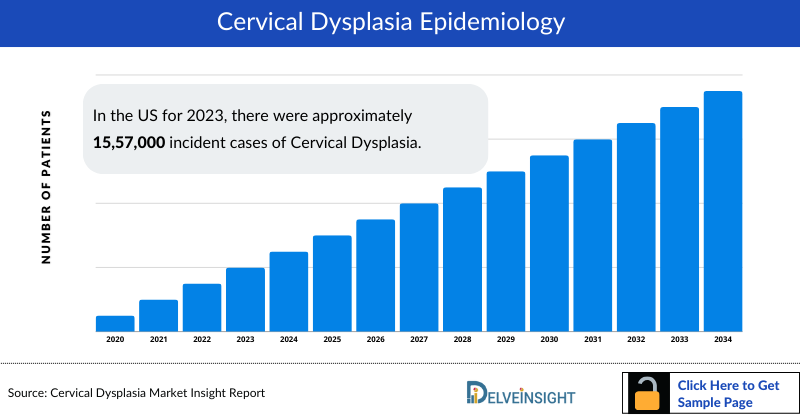
The Cervical Dysplasia epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by total diagnosed incident cases of Cervical Dysplasia, total diagnosed incident cases for Cervical Dysplasia by severity (without the year-on-year impact of vaccination), age group-specific cases (CIN 2+, with the impact of HPV vaccination), total treated cases of cervical dysplasia in the 7MM covering the United States, EU4 countries (Germany, France, Italy, and Spain), United Kingdom, and Japan from 2020 to 2034.
- In the US for 2023, there were approximately 15,57,000 incident cases of Cervical Dysplasia.
- Among EU4 and the UK, France accounted for the highest number of cases of Cervical Dysplasia with dominance in CIN-1 severity-specific cases.
Cervical Dysplasia Drug Chapters
The drug chapter segment of the Cervical Dysplasia report encloses a detailed analysis of Cervical Dysplasia marketed drugs and late-stage (Phase III and Phase II) pipeline drugs. It also deep dives into the Cervical Dysplasia pivotal clinical trial details, recent and expected market approvals, patent details, the latest news, and recent deals and collaborations.
Emerging Drugs
Artesunate: Frantz Viral Therapeutics
Artesunate is a semi-synthetic derivative of artemisinin, made by the plant sweet wormwood (Artemisia annua). This Chinese herbal medicine has been used for centuries to treat malaria and now is WHO-approved as a first-line treatment for acute malaria. Its safety profile has been well-documented in treating millions of individuals with acute malaria ranging from infants to adults. The artesunate therapy is self-administered, thus addressing treatment resource limitations. Self-administered artesunate vaginal inserts eliminate both CIN2/3 lesions as well as HPV. The artesunate treatment provides a nonsurgical, safe therapy within the standard of care window, still allowing for excision/ablation procedures, if necessary, at the end of the window.
Currently, it is in Phase IIb trial for artesunate vaginal inserts for the treatment of patients with cervical intraepithelial neoplasia 2/3.
Note: Detailed emerging therapies assessment will be provided in the final report.
|
Therapy Name |
Company Name |
ROA |
MOA |
Phases |
Molecule Type |
|
Artesunate |
Frantz Viral Therapeutics |
Intravaginal |
Cell inhibitors |
IIb |
Small Molecule |
|
VGX-3100 |
Inovio Pharmaceuticals |
Intramuscular |
Immunostimulants |
III |
DNA Vaccines |
Cervical Dysplasia Market Outlook
A few potential therapies are being investigated for the treatment of Cervical Dysplasia.
- • Among the 7MM, the United States accounts for the largest market share.
- • Among EU4 and the UK, Germany and France account for the largest market share.
Key players, such as Frantz Viral Therapeutics, and others are evaluating their lead candidates in different stages of clinical development, respectively. They aim to investigate their products for the treatment of Cervical Dysplasia.
Cervical Dysplasia Drugs Uptake
This section focuses on the uptake rate of potential drugs expected to be launched in the market during 2020–2034, which depends on the competitive landscape, safety, and efficacy data along with order of entry. It is important to understand that the key players evaluating their novel therapies in the pivotal and confirmatory trials should remain vigilant when selecting appropriate comparators to stand the greatest chance of a positive opinion from regulatory bodies, leading to approval, smooth launch and rapid uptake.
Further detailed analysis of emerging therapies drug uptake in the report…
Cervical Dysplasia Activities
The report provides insights into different therapeutic candidates in Phase III and Phase II stages. It also analyzes key players involved in developing targeted therapeutics.
Pipeline Development Activities
The report covers information on collaborations, acquisitions and mergers, licensing, and patent details for Cervical Dysplasia emerging therapies.
KOL Views
To keep up with the real-world scenario in current and emerging market trends, we take opinions from Key Industry leaders working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts contacted for insights on evolving treatment landscape, patient reliance on conventional therapies, patient’s therapy switching acceptability, and drug uptake along with challenges related to accessibility.
Delveinsight’s analysts connected with 40+ KOLs to gather insights; however, interviews were conducted with 18+ KOLs in the 7MM. Their opinion helps understand and validate current and emerging therapy treatment patterns for Cervical Dysplasia.
Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT analysis and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of gaps in disease diagnosis, patient awareness, physician acceptability, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided.
Conjoint Analysis analyzes multiple approved and emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
In efficacy, the trial’s primary and secondary outcome measures are evaluated; for instance, in event-free survival, one of the most important primary outcome measures is event-free survival and overall survival.
Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Market Access and Reimbursement
Health technology assessments (HTAs) evaluate published literature according to evidence-based criteria to present a high-quality scientific synthesis of available evidence regarding clinical benefits, harms, economic consequences, and ethical or social issues. HTAs are increasingly leveraged as an essential requirement for healthcare decision-making. Ideally, an HTA is expected to evaluate the long-term benefits and risks of a given medical intervention to its costs.
The report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of approved therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Scope of the Report
- The report covers a segment of key events, an executive summary, descriptive overview of Cervical Dysplasia, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight has been provided into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, and disease progression along treatment guidelines.
- Additionally, an all-inclusive account of both the current and emerging therapies, along with the elaborative profiles of late-stage and prominent therapies, will have an impact on the current treatment landscape.
- A detailed review of the Cervical Dysplasia market, historical and forecast market size, market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help in shaping and driving the 7MM Cervical Dysplasia.
Cervical Dysplasia Report Insights
- Patient Population
- Therapeutic Approaches
- Cervical Dysplasia Pipeline Analysis
- Cervical Dysplasia Market Size and Trends
- Existing and future Market Opportunity
Cervical Dysplasia Report Key Strengths
- Ten Years Forecast
- 7MM Coverage
- Cervical Dysplasia Epidemiology Segmentation
- Key Cross Competition
- Conjoint analysis
- Drugs Uptake and Key Market Forecast Assumptions
Cervical Dysplasia Report Assessment
- Current Treatment Practices
- Unmet Needs
- Pipeline Product Profiles
- Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
FAQs
- What is the historical and forecast Cervical Dysplasia patient pool/patient buden in the United States, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan?
- What was the Cervical Dysplasia total market size, the market size by therapies, market share (%) distribution in 2020, and what would it look like in 2034? What are the contributing factors/key catalysts for this growth?
- Which combination treatment approaches will have a significant impact on Cervical Dysplasia drug treatment market size?
- Is there any unexplored patient setting that can open the window for growth in the future?
- Which class is going to be the largest contributor in market size in 2034?
- What are major changes in new treatment guidelines and impact on future treatment landscape?
- What are the pricing variations among different geographies for approved therapies?
- How would the market drivers, barriers, and future opportunities affect the market dynamics and subsequent analysis of the associated trends?
- What are the current and emerging options for the treatment of Cervical Dysplasia?
- How many companies are developing therapies for the treatment of Cervical Dysplasia?
- What are the recent novel therapies, targets, mechanisms of action, and technologies developed to overcome the limitation of existing therapies?
- Patient acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the country-specific accessibility issues of expensive, recently approved therapies?
Reasons to buy
- The report will help in developing business strategies by understanding the latest trends and changing treatment dynamics driving the Cervical Dysplasia Market.
- Insights on patient burden/disease prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years
- Understand the existing market opportunity in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Patient-based forecast model which uses bottom-up forecasting techniques is accepted as a gold standard in pharma forecasting.
- Identifying strong upcoming players in the market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the Conjoint analysis section to provide visibility around leading classes.
- Highlights of access and reimbursement policies of approved therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet need of the existing market so that the upcoming players can strengthen their development and launch strategy.