Chemotherapy Induced Diarrhea Market
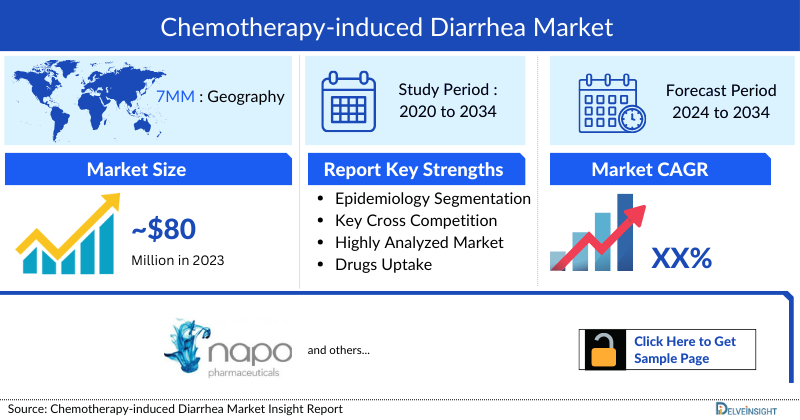
- The Chemotherapy Induced Diarrhea Market Size in the 7MM was around USD 80 million in 2023.
- The United States accounts for the largest market size (around USD 40 million) of Chemotherapy Induced Diarrhea, in comparison to EU4 (Germany, Spain, Italy, France), the United Kingdom, and Japan.
- Chemotherapy Induced Diarrhea, also called chemotherapy-related diarrhea, is a common problem in cancer patients and is most often described with fluoropyrimidines, irinotecan, and several molecularly targeted agents.
- Chemotherapy –induced diarrhea, specially Grade III-IV may cause dose reduction, treatment delay or treatment discontinuation in as high as 70% of patients who are prescribed chemotherapy
- The cancers included in the report are colorectal cancer, breast cancer, bladder cancer, cervical cancer, esophageal cancer, head and neck, lung cancer, ovarian cancer, and 5 more
- Approximately 60% of these cancer incident cases receive chemotherapy.
- It is estimated that lung cancer contributed to the highest number of chemotherapy-related diarrhea cases in the United States, nearly 30%.
- The market size of Chemotherapy Induced Diarrhea in the seven major markets was around USD 80 million in 2023. The market is anticipated to witness a substantial positive shift owing to an increase in the number of cancer patients thus more patients seeking chemotherapy as a part of their treatment and encountering side effects like Chemotherapy Induced Diarrhea.
- In EU4 and the UK, approximately 20% of Grade I-II chemotherapy-related diarrhea patients do not respond to first-line therapy and are considered refractory.
- In 2023, total number of non-refractory and refractory treated cases of chemotherapy-related diarrhea was ~320,000 and ~40,000 in the US.
- Napo Pharmaceutical’s MYTESI (crofelemer) is the only therapy under development for the prevention of chemotherapy-related diarrhea in the 7MM. This drug is currently marketed for non‐infectious diarrhea in adult patients with HIV/AIDS on anti‐retroviral therapy.
- If approved, MYTESI (crofelemer) can prove to be an important prophylactic agent and help cancer patients mitigate one of the treatments limiting morbidity and improving their QoL.
Request for unlocking the Sample Page of the "Chemotherapy Induced Diarrhea Treatment Market"
DelveInsight's “Chemotherapy Induced Diarrhea Market Insights, Epidemiology and Market Forecast – 2034” report delivers an in-depth understanding of Chemotherapy Induced Diarrhea, historical and forecasted epidemiology as well as the Chemotherapy Induced Diarrhea market trends in the United States, EU4 (Germany, Spain, Italy, and France) and the United Kingdom, and Japan.
Chemotherapy Induced Diarrhea Treatment Market report provides real-world prescription pattern analysis, emerging drugs, market share of individual therapies, and historical and forecasted 7MM Chemotherapy Induced Diarrhea market size from 2020 to 2034. The report also covers current Chemotherapy Induced Diarrhea treatment market practices/algorithms and unmet medical needs to curate the best opportunities and assess the market’s underlying potential.
|
Study Period |
2020 to 2034 |
|
Forecast Period |
2024-2034 |
|
Geographies Covered |
|
|
Chemotherapy Induced Diarrhea Market |
|
|
Chemotherapy Induced Diarrhea Market Size |
USD 80 Million in 2023 |
|
Chemotherapy Induced Diarrhea Companies |
|
Chemotherapy Induced Diarrhea Treatment Market: Understanding and Algorithm
Most patients with cancer receive curative or palliative chemotherapeutic intervention throughout their treatment course. Gastrointestinal toxicities, including nausea, vomiting, ulceration, bleeding, constipation, and diarrhea, are often the major causes of treatment delays, dose adjustment, and treatment discontinuation during chemotherapy. Diarrhea is an unpleasant but common side effect in people receiving treatment for cancer, or cancer itself may cause it. The duration and severity of diarrhea may depend on the factors causing it; sometimes, it can signify something more serious. It is very common or normal to have diarrhea for anyone, but for a cancer patient, there may be other causes than what commonly causes diarrhea, such as:
- Cancer treatment
- Infections
- Cancer itself
The severity is often described using the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) grades. Severity is determined by the number of stools per day or increased ostomy output compared with baseline, the need for hospitalization, and the effect on self-care activities. It is critical to ascertain the patient’s baseline bowel pattern when grading the severity of chemotherapy-related diarrhea.
The Chemotherapy Induced Diarrhea report provides an overview of Chemotherapy Induced Diarrhea pathophysiology, diagnostic approaches, and detailed treatment algorithm along with a real-world scenario of a patient’s journey beginning from the first symptom, the time taken for diagnosis to the entire treatment process.
Chemotherapy Induced Diarrhea Treatment
Treatment for chemotherapy-related diarrhea includes nonpharmacologic and pharmacologic interventions to slow diarrhea and careful serial evaluation to assess therapy response and rule out significant volume depletion or other risk factors that would require targeted intervention or hospitalization. Initial management depends on the severity of diarrhea and whether or not additional “risk factors” are present. The management is recommended based on classifying patients with “uncomplicated” or “complicated.”
The mainstays of pharmacologic therapy for chemotherapy-related diarrhea are opioids. IMODIUM (Loperamide) and LOMOTIL (diphenoxylate-atropine) are the most commonly used, and both are US FDA-approved for this diarrhea; both have a rapid onset of action. Initial nonpharmacologic measures include avoidance of foods that might aggravate diarrhea and aggressive oral rehydration with fluids that contain water, salt, and sugar (since glucose promotes intestinal sodium absorption).
Chemotherapy Induced Diarrhea Epidemiology
The Chemotherapy Induced Diarrhea epidemiology chapter in the report provides historical as well as forecasted in the 7MM covering the United States, EU4 countries (Germany, France, Italy, and Spain), the United Kingdom, and Japan from 2024 to 2034. The Chemotherapy Induced Diarrhea epidemiology is segmented with detailed insights into incident cases of selected cancer types, total patients on chemotherapies by cancer type, incident cases of CID by selected cancer types, grade-specific incident cases of CID, and total treated cases of CID.
- According to the findings, diarrhea in patients with cancer is extremely common, with approximately 50–80% of those on chemotherapy suffering from diarrhea. Among patients treated with chemotherapy, about 30% of those cases are severe.
- According to DelveInsight's estimates, around 30% of breast cancer patients in the United States were undergoing chemotherapy as part of their treatment regimen in 2023.
- According to DelveInsight's estimates, Grade I–II chemotherapy-related diarrhea accounted for nearly 60,000 incident cases in Germany in 2023, the highest among the EU4 and the UK.
Unlock comprehensive insights! Click Here to Purchase the Full Epidemiology Report @ Chemotherapy Induced Diarrhea Prevalence
Chemotherapy Induced Diarrhea Drug Chapters
The drug chapter segment of the Chemotherapy Induced Diarrhea report encloses a detailed analysis of Chemotherapy Induced Diarrhea marketed drugs and late-stage (Phase III and Phase II) Chemotherapy Induced Diarrhea pipeline drugs. It also deep dives into Chemotherapy Induced Diarrhea pivotal clinical trial details, recent and expected market approvals, patent details, the latest news, and recent deals and collaborations.
Chemotherapy Induced Diarrhea Marketed Drugs
The mainstays of pharmacologic therapy for Chemotherapy Induced Diarrhea are opioids. IMODIUM (Loperamide) and LOMOTIL (diphenoxylate-atropine) are the most commonly used, and both are US FDA-approved for diarrhea; both have a rapid onset of action. Currently, there are no approved drugs for Chemotherapy Induced Diarrhea.
Note: Detailed current therapies assessment will be provided in the full report of Chemotherapy Induced Diarrhea...
Chemotherapy Induced Diarrhea Emerging Drugs
- MYTESI (crofelemer): Napo Pharmaceuticals /Jaguar Health
The current cornerstone of management of diarrhea and dehydration, oral rehydration solution (ORS), does not affect the severity or duration of diarrhea. Crofelemer is an investigational drug developed by Napo Pharmaceuticals, a wholly-owned subsidiary of Jaguar Health in California, to treat secretory diarrhea from various causes. It is a novel, first-in-class antisecretory agent that normalizes electrolyte and fluid balance while acting locally in the gut. This mechanism of action can benefit multiple disorders that cause gastrointestinal distress, including diarrhea and abdominal discomfort.
This antidiarrheal agent is an isolated and purified compound extracted from the red bark sap, also referred to as “dragon’s blood,” of the Croton lechleri (Euphorbiaceae) tree. Napo Pharmaceuticals has completed a Phase II clinical trial (NCT02910219) for crofelemer in diarrhea prevention and prophylaxis with crofelemer in HER2-positive breast cancer patients receiving trastuzumab, pertuzumab, and docetaxel or paclitaxel with or without carboplatin, which was completed in 2020. The company has started investigating the drug in Phase III clinical trial (NCT04538625), estimated to be completed in 2023.
Note: Detailed emerging therapies assessment will be provided in the final report.
Chemotherapy Induced Diarrhea Market Outlook
Despite the high incidence of Chemotherapy Induced Diarrhea, currently, there is no effective Chemotherapy Induced Diarrhea treatment for humans available for use in the market. There is also a high unmet need for therapies to treat chemotherapy-related diarrhea. Currently, there are no FDA-approved drugs in the chemotherapy-related diarrhea market.
Nevertheless, Napo Pharmaceuticals, a wholly-owned subsidiary of Jaguar Health, has initiated Chemotherapy Induced Diarrhea clinical trials investigating new treatment options. The company is investigating its candidate, MYTESI (crofelemer), to manage Chemotherapy Induced Diarrhea in the 7MM.
- The Chemotherapy Induced Diarrhea Market Size in the seven major markets was around USD 80 million in 2023.
- The United States accounts for the largest Chemotherapy Induced Diarrhea Market Size (around USD 40 million) of Chemotherapy Induced Diarrhea, in comparison to EU4 (Germany, Spain, Italy, France), the United Kingdom, and Japan.
- Japan accounts for the second highest Chemotherapy Induced Diarrhea Market Size in the 7MM during the forecast period 2024–2034.
- Among EU4 and the UK, Germany has the highest Chemotherapy Induced Diarrhea market size.
Chemotherapy Induced Diarrhea Drugs Uptake
This section focuses on the uptake rate of potential Chemotherapy Induced Diarrhea drugs expected to be launched in the market during 2024–2034, which depends on the competitive landscape, safety, and efficacy data along with order of entry. It is important to understand that the key players evaluating their novel therapies in the pivotal and confirmatory trials should remain vigilant when selecting appropriate comparators to stand the greatest chance of a positive opinion from regulatory bodies, leading to approval, smooth launch, and rapid uptake.
Further detailed analysis of emerging therapies drug uptake in the report...
Chemotherapy Induced Diarrhea Activities
The Chemotherapy Induced Diarrhea therapeutics market report provides insights into different therapeutic candidates in Phase III and Phase II stages. It also analyzes key Chemotherapy Induced Diarrhea Companies involved in developing targeted therapeutics.
Pipeline Development Activities
The Chemotherapy Induced Diarrhea therapeutics market report covers information on collaborations, acquisitions and mergers, licensing, and patent details for Chemotherapy Induced Diarrhea emerging therapies.
Take Your Research to the Next Level! Click Here to Get Access to the Full Pipeline Report @ Chemotherapy Induced Diarrhea Treatment Drugs
KOL Views
To keep up with the real-world scenario in current and emerging market trends, we take opinions from Key Industry leaders working in the domain through primary research to fill the data gaps and validate our secondary research. Industry Experts were contacted for insights on the evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake along with challenges related to accessibility. DelveInsight’s analysts connected with 10+ KOLs to gather insights; however, interviews were conducted with 5+ KOLs in the 7MM. Their opinion helps us understand and validate current and emerging treatment patterns for Chemotherapy Induced Diarrhea. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
KOL Views
“Effectively treating diarrhea in patients with cancer requires a robust and holistic assessment of patients, both at presentation and during their treatment journey. It is not just a matter of evaluating one single symptom; one needs to assess the overall symptoms the patient is experiencing.” “Baseline assessment should include — at a minimum — full blood cell count, biochemical examination, stool microscopy, and culture for drug-resistant organisms. All the possible causes of superimposed diarrhea have to be defined.”“There is an urgent need for new supportive therapies to help cancer patients manage chemotherapy-related diarrhea. The side effects of cancer therapies can be so debilitating for patients that they either cannot maintain effective doses of treatment or choose to discontinue treatment altogether.”
|
KOL Views |
|
“Effectively treating diarrhea in patients with cancer requires a robust and holistic assessment of patients, both at presentation and during their treatment journey. It is not just a matter of evaluating one single symptom; one needs to assess the overall symptoms the patient is experiencing.” |
|
“Baseline assessment should include — at a minimum — full blood cell count, biochemical examination, stool microscopy, and culture for drug-resistant organisms. All the possible causes of superimposed diarrhea have to be defined.” |
|
“There is an urgent need for new supportive therapies to help cancer patients manage chemotherapy-related diarrhea. The side effects of cancer therapies can be so debilitating for patients that they either cannot maintain effective doses of treatment or choose to discontinue treatment altogether.” |
Qualitative Analysis
We perform Qualitative and Chemotherapy Induced Diarrhea Treatment Market Intelligence analysis using various approaches, such as SWOT analysis and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of gaps in disease diagnosis, patient awareness, physician acceptability, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. Conjoint Analysis analyzes multiple approved and emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
In efficacy, the trial’s primary and secondary outcome measures are evaluated; for instance, in event-free survival, one of the most important primary outcome measures is event-free survival and overall survival. Further, the therapies’ safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Chemotherapy Induced Diarrhea Treatment Market Access and Reimbursement
The Chemotherapy Induced Diarrhea treatment market report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of currently used therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Chemotherapy Induced Diarrhea Treatment Market Report Scope
- The Chemotherapy Induced Diarrhea drugs market report covers a segment of key events, an executive summary, descriptive overview, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight has been provided into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, and disease progression along with country-specific Chemotherapy Induced Diarrhea treatment market guidelines.
- Additionally, an all-inclusive account of both the current and emerging therapies, along with the elaborative profiles of late-stage and prominent therapies, will have an impact on the current Chemotherapy Induced Diarrhea treatment market landscape.
- A detailed review of the Chemotherapy Induced Diarrhea treatment market, historical and forecasted market size, Chemotherapy Induced Diarrhea market share by therapies, detailed assumptions, and rationale behind our approach is included in the report, covering the 7MM drug outreach.
- The Chemotherapy Induced Diarrhea treatment market report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help in shaping and driving the 7MM Chemotherapy Induced Diarrhea drugs market.
Chemotherapy Induced Diarrhea Treatment Market Report Insights
- Patient-based Chemotherapy Induced Diarrhea Market Forecasting
- Chemotherapy Induced Diarrhea Therapeutic Approaches
- Chemotherapy Induced Diarrhea Pipeline Analysis
- Chemotherapy Induced Diarrhea Market Size and Trends
- Existing and future Chemotherapy Induced Diarrhea Treatment Market Opportunity
Chemotherapy Induced Diarrhea Treatment Market Report Key Strengths
- 10 Years- Chemotherapy Induced Diarrhea Market Forecast
- 7MM Coverage
- Chemotherapy Induced Diarrhea Epidemiology Segmentation
- Inclusion of Country specific Chemotherapy Induced Diarrhea Treatment Market guidelines
- KOL’s feedback on approved and emerging therapies
- Key Cross Competition
- Conjoint analysis
- Chemotherapy Induced Diarrhea Drugs Uptake
- Key Chemotherapy Induced Diarrhea Market Forecast Assumptions
Chemotherapy Induced Diarrhea Treatment Market Report Assessment
- Current Chemotherapy Induced Diarrhea Treatment Market Practices
- Chemotherapy Induced Diarrhea Unmet Needs
- Chemotherapy Induced Diarrhea Pipeline Product Profiles
- Chemotherapy Induced Diarrhea Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
- Chemotherapy Induced Diarrhea Market Drivers
- Chemotherapy Induced Diarrhea Market Barriers
FAQs
- What is the growth rate of the 7MM Chemotherapy Induced Diarrhea treatment market?
- What was the Chemotherapy Induced Diarrhea market size, the Chemotherapy Induced Diarrhea market size by therapies, Chemotherapy Induced Diarrhea market share (%) distribution in 2020, and what would it look like in 2034? What are the contributing factors/key catalysts for this growth?
- Is there any unexplored patient setting that can open the window for growth in the future?
- What are the pricing variations among different geographies for approved and off-label therapies?
- How would the market drivers, barriers, and future opportunities affect the market dynamics and subsequent analysis of the associated trends? Although multiple expert guidelines recommend testing for targetable mutations before therapy initiation, why do barriers to testing remain high?
- What are the current and emerging options for the treatment of Chemotherapy Induced Diarrhea?
- How many companies are developing therapies for the treatment of Chemotherapy Induced Diarrhea?
- What are the recent novel therapies, targets, mechanisms of action, and technologies developed to overcome the limitations of existing therapies?
- Patient/physician acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the country-specific accessibility issues of expensive, recently approved therapies?
Reasons to Buy
- TheChemotherapy Induced Diarrhea treatment market report will help in developing business strategies by understanding the latest trends and changing treatment dynamics driving the Chemotherapy Induced Diarrhea Therapeutics Market.
- Insights on patient burden/disease Chemotherapy Induced Diarrhea prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years
- Understand the existing Chemotherapy Induced Diarrhea Therapeutics Market opportunities in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identifying strong upcoming players in the Chemotherapy Induced Diarrhea Therapeutic Market will help devise strategies to help get ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the conjoint analysis section to provide visibility around leading classes.
- Highlights of access and reimbursement policies of approved therapies, barriers to accessibility of expensive off-label therapies, and patient assistance programs.
- To understand Key Opinion Leaders’ perspectives around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing Chemotherapy Induced Diarrhea treatment market so that the upcoming players can strengthen their development and launch strategy.
Stay Updated with us for Recent Articles