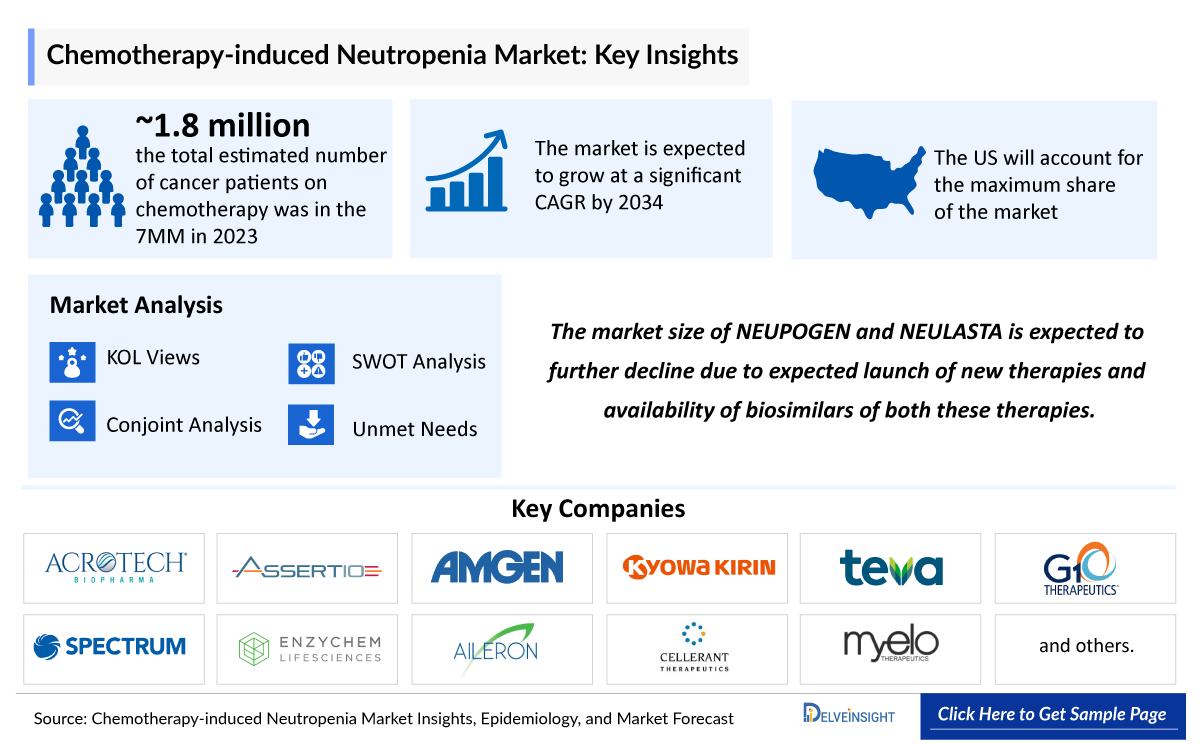
Chemotherapy Induced Neutropenia Market Summary
- The Chemotherapy-Induced Neutropenia Market Size is projected to grow steadily from 2025 to 2034, driven by rising cancer incidence, increased use of myelosuppressive chemotherapy, and greater awareness of infection risks. Advances in long-acting granulocyte colony-stimulating factors (G-CSF), prophylactic antimicrobials, and precision risk assessment, supported by ongoing research, are expected to enhance patient management and drive market expansion.
- The leading Chemotherapy Induced Neutropenia Companies include - Acrotech Biopharma, Assertio Holdings, Amgen, Kyowa Hakko Kirin Co., Ltd., Teva B.V., G1 Therapeutics, Spectrum Pharmaceuticals, Enzychem Lifesciences Corporation, Aileron Therapeutics, Cellerant Therapeutics, Myelo therapeutics, BeyondSpring Pharmaceuticals, Evive Biotech, Spectrum Pharmaceuticals, and others.
Chemotherapy Induced Neutropenia Market & Epidemiology Analysis
- Myelosuppressive chemotherapy in cancer treatment increases the risk of chemotherapy-induced neutropenia, which can lead to serious complications such as febrile neutropenia, hospitalization, and higher mortality rates.
- Additionally to manage neutropenia, oncologists may reduce chemotherapy doses, resulting in lower relative dose intensity (RDI) and potentially compromising treatment outcomes.
- The approvals of ROLVEDON and RYZNEUTA represent important progress in G-CSF therapies for chemotherapy-induced neutropenia, providing long-acting, patient-centered options that improve infection prevention and help maintain continuity of cancer treatment.
- The Chemotherapy-induced neutropenia pipeline remains relatively limited, reflecting ongoing reliance on supportive care over innovative therapies. Most progress centers on optimizing existing G-CSFs, with few novel or targeted agents emerging. This highlights a significant unmet need for differentiated treatments, especially for high-risk or treatment-refractory patients.
- Market growth in chemotherapy-induced neutropenia is driven by increasing cancer incidence and expanded use of myelosuppressive chemotherapy. Progress in supportive care, including long-acting G-CSFs, novel antimicrobials, and precision-based risk assessment, is improving patient outcomes, although the rising threat of antimicrobial resistance remains a significant management challenge.
Request for Unlocking the Sample Page of the "Chemotherapy-Induced Neutropenia Market Insights"
Key Factors Driving the Chemotherapy-Induced Neutropenia Market
- Rising cancer incidence and chemotherapy utilization: The growing global cancer burden has increased the use of myelosuppressive chemotherapy, expanding the patient population at risk of chemotherapy-induced neutropenia.
- Greater focus on infection prevention and supportive care: Increased awareness of febrile neutropenia–related complications has driven proactive use of prophylactic and therapeutic agents to reduce infection risk.
- Advancements in G-CSF and biosimilar therapies: The availability of long-acting formulations and cost-effective biosimilars has improved access and adoption across both hospital and outpatient settings.
- Stronger clinical guidelines and risk-based treatment approaches: Updated oncology guidelines recommending early intervention for high-risk patients are supporting consistent use of neutropenia management therapies.
- Improving healthcare access in emerging markets: Expanding oncology infrastructure and better reimbursement for supportive care drugs in emerging economies are contributing to market growth.
DelveInsight’s comprehensive report titled “Chemotherapy-induced Neutropenia Treatment Market Insights, Epidemiology, and Market Forecast – 2034” offers a detailed analysis of Chemotherapy-induced Neutropenia. The report presents historical and projected epidemiological data covering total incident cases of chemotherapy-induced neutropenia, gender-specific cases of chemotherapy-induced neutropenia, severity-specific cases of chemotherapy-induced neutropenia, and treated cases of chemotherapy-induced neutropenia. In addition to epidemiology, the market report encompasses various aspects related to the patient population. These aspects include the diagnosis process, prescription patterns, physician perspectives, market accessibility, treatment options, and prospective developments in the market across seven major markets: the United States, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan, spanning from 2020 to 2034.
The Chemotherapy Induced Neutropenia Treatment Market Report analyzes the existing treatment practices and unmet medical requirements in Chemotherapy-induced Neutropenia. It evaluates the market potential and identifies potential business prospects for enhancing therapies or interventions. This valuable information enables stakeholders to make well-informed decisions regarding product development and strategic planning for the market.
Scope of the Chemotherapy Induced Neutropenia Market | |
|
Study Period |
2020 to 2034 |
|
Forecast Period |
2025-2034 |
|
Geographies Covered |
|
|
Chemotherapy-Induced Neutropenia Market |
|
|
Chemotherapy-Induced Neutropenia Market Size | |
|
Chemotherapy-Induced Neutropenia Companies |
Acrotech Biopharma, Assertio Holdings, Amgen, Kyowa Hakko Kirin Co., Ltd., Teva B.V., G1 Therapeutics, Spectrum Pharmaceuticals, Enzychem Lifesciences Corporation, Aileron Therapeutics, Cellerant Therapeutics, Myelo therapeutics, BeyondSpring Pharmaceuticals, Evive Biotech, Spectrum Pharmaceuticals, and others |
|
Chemotherapy-Induced Neutropenia Epidemiology Segmentation |
|
Chemotherapy-induced Neutropenia Disease Understanding
Chemotherapy-induced Neutropenia Overview
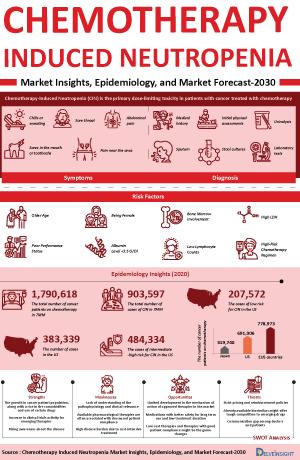
Chemotherapy-induced neutropenia is a common and clinically significant adverse effect of cytotoxic treatment, contributing to considerable morbidity and mortality. It markedly increases susceptibility to infections, with febrile neutropenia representing the most severe consequence. This complication often necessitates chemotherapy dose reductions or delays, potentially compromising treatment outcomes. Hospitalization is frequently required, with infection-related complications posing a substantial risk of death. In addition, the condition imposes a high economic burden due to greater reliance on antibiotics and unplanned inpatient care.
Clinical consequences of chemotherapy-induced neutropenia include febrile neutropenia, increased use of oral or intravenous antibiotics, unplanned emergency visits, hospital admissions, and potential mortality. Additionally, subsequent chemotherapy cycles may require dose reductions or delays to manage neutropenia, which can negatively affect treatment outcomes.
Chemotherapy-induced Neutropenia Diagnosis
The diagnosis of chemotherapy-induced neutropenia begins with a complete blood count to confirm neutropenia and assess severity, along with a peripheral blood smear to rule out pseudoneutropenia or other blood abnormalities. Cultures from blood, urine, sputum, or other sites are taken to identify infection sources, especially in febrile patients. Imaging like chest X-rays devices and computed tomography (CT) scans help detect infections such as pneumonia or abdominal issues. Bone marrow biopsy is reserved for cases with delayed recovery or suspected marrow disorders.
Routine immunological tests and genetic studies are generally unnecessary unless there are signs of primary immunodeficiency or autoimmune causes. Additional evaluations may include blood chemistry, viral infection screening, nutritional assessments, and tumor markers when clinically indicated.
Chemotherapy-induced Neutropenia Treatment Algorithim
G-CSFs, such as filgrastim, pegfilgrastim, and newer agents like eflapegrastim (ROLVEDON), are the mainstay of chemotherapy-induced neutropenia management. They stimulate neutrophil production to reduce the severity and duration of neutropenia, lowering the risk of infections. G-CSFs are used both to prevent neutropenia in high-risk patients and to treat it after febrile episodes, with dosing tailored to individual patient needs. At the onset of febrile neutropenia, empirical broad-spectrum intravenous antibiotics should be started promptly, with oral antibiotics reserved for low-risk cases. Antifungal and antiviral treatments are used in prolonged or confirmed infections.
To prevent recurrence, chemotherapy doses may be delayed or reduced, balancing the need for effective cancer treatment with patient safety. Supportive care includes hydration, electrolyte management, and close infection monitoring. Infection prevention strategies involve prophylactic antibiotics, antifungals, and vaccinations against common pathogens like influenza. Hematopoietic stem cell transplant is generally not used for chemotherapy-induced neutropenia unless there is an underlying bone marrow disorder or malignancy.
Chemotherapy-induced Neutropenia Epidemiology
The epidemiology section of the chemotherapy-induced neutropenia market report offers information on the patient populations, including historical and projected trends for each of the seven major markets. Examining key opinion leader views from physicians or clinical experts can assist in identifying the reasons behind historical and projected trends. The diagnosed patient pool, their trends, and the underlying assumptions are all included in this section of the report. This section also presents the Chemotherapy-Induced Neutropenia Prevalence Data with relevant tables and graphs, offering a clear and concise view of the incidence of chemotherapy-induced neutropenia. Additionally, the report discloses the assumptions made during the analysis, ensuring data interpretation and presentation transparency. This epidemiological data is valuable for understanding the disease burden and its impact on the patient population across various regions.
Key Findings from the Chemotherapy Induced Neutropenia Epidemiological Forecast
- As per secondary source, the risk of developing febrile neutropenia during chemotherapy for solid tumors is estimated at around 17%.
- According to the secondary findings, febrile neutropenia develops in about 13–21% of patients undergoing standard myelosuppressive chemotherapy for metastatic solid tumors, with the highest incidence typically seen in the first treatment cycle.
- The epidemiology of chemotherapy-induced neutropenia is expected to change during the forecast period (2025-2034).
Chemotherapy-induced Neutropenia Epidemiology Segmentation
- Total Chemotherapy-Induced Neutropenia Incident Cases
- Total Chemotherapy-Induced Neutropenia Gender-specific Cases
- Total Chemotherapy-Induced Neutropenia Severity-specific Cases
- Total Chemotherapy-Induced Neutropenia Treated Cases
Chemotherapy-induced Neutropenia Drug Analysis
The drug chapter segment of the Chemotherapy-induced Neutropenia drugs market report encloses the detailed analysis of Chemotherapy-induced Neutropenia marketed drugs and late-stage (Phase-III and Phase-II) Chemotherapy-induced Neutropenia pipeline drugs. It also helps to understand the Chemotherapy-induced Neutropenia clinical trial details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug, and the latest news and press releases.
Chemotherapy-induced Neutropenia Marketed Drugs
ROLVEDON (eflapegrastim-xnst): Assertio Holdings/Spectrum Pharmaceuticals
ROLVEDON (eflapegrastim-xnst) injection is a long-acting G-CSF with a novel formulation. Spectrum has received an indication to decrease the incidence of infection, as manifested by febrile neutropenia, in adult patients with non-myeloid malignancies receiving myelosuppressive anti-cancer drugs associated with clinically significant incidence of febrile Neutropenia.
- In September 2022, Spectrum Pharmaceuticals, announced that the US FDA has approved ROLVEDON (eflapegrastim-xnst) injection to decrease the incidence of infection, as manifested by febrile Neutropenia, in adult patients with non-myeloid malignancies receiving myelosuppressive anti-cancer drugs associated with clinically significant incidence of febrile Neutropenia.
- In July 2023, Assertio Holdings has announced the completion of its planned acquisition of Spectrum Pharmaceuticals.
RYZNEUTA (efbemalenograstim alfa-vuxw): Evive Biotechnology/Acrotech Biopharma
RYZNEUTA is a leukocyte growth factor indicated to decrease the incidence of infection, as manifested by Chemotherapy-induced Neutropenia, in adult patients with non-myeloid malignancies receiving myelosuppressive anti-cancer drugs associated with a clinically significant incidence of Chemotherapy-induced Neutropenia. RYZNEUTA is the first non-pegylated granulocyte colony-stimulating factor approved by both the US FDA.
- In November 2023, Evive Biotech and Acrotech Biopharma announced that the US FDA has approved RYZNEUTA (efbemalenograstim alfa) for the reduction of infection risk, as indicated by chemotherapy-induced Neutropenia, in adult patients with non-myeloid cancers undergoing myelosuppressive chemotherapy associated with a clinically significant risk of chemotherapy-induced neutropenia.
- In March 2024, Evive Biotech announced European Commission approval of RYZNEUTA (efbemalenograstim alfa injection) for chemotherapy-induced neutropenia.
|
Drug |
MoA |
RoA |
Company |
Logo |
|
ROLVEDON (eflapegrastim-xnst) |
Long-acting G-CSF analog |
Subcutaneous injection |
Assertio/ Spectrum Pharmaceuticals |
|
|
RYZNEUTA (efbemalenograstim alfa-vuxw) |
Colony-stimulating factor |
Subcutaneous injection |
Evive Biotechnology/Acrotech Biopharma |
|
|
XX |
XX |
X |
XXX |
|
Note: Detailed marketed therapies assessment will be provided in the final report...
Chemotherapy-induced Neutropenia Market Outlook
The Chemotherapy-induced Neutropenia therapeutics market is further expected to increase by the major drivers, such as the rising incidence population, technological advancements, and upcoming therapies in the forecast period (2025–2034). With ongoing research and continued dedication, the future holds hope for even more effective treatments and, ultimately, a cure for this challenging condition. According to DelveInsight, the Chemotherapy-induced Neutropenia market in the 7MM is expected to change significantly during the forecast period 2025–2034.
Chemotherapy-induced Neutropenia Competitive Landscape
The competitive landscape for chemotherapy-induced neutropenia is defined by established biologics and emerging biosimilars, with key players focusing on granulocyte colony-stimulating factors (G-CSFs) to prevent and treat neutropenia. Market competition centers on long-acting and more cost-effective formulations that improve patient convenience and adherence. Strategic activities such as product launches, patent expirations, partnerships, and regional market expansions are shaping competition. Differentiation is driven by clinical efficacy, safety profiles, dosing convenience, pricing, and reimbursement access, as stakeholders vie to capture growth in both developed and emerging oncology care settings.
Key Chemotherapy-induced Neutropenia Companies
The Key Chemotherapy-induced Neutropenia companies actively involved in the Chemotherapy-induced Neutropenia treatment landscape include -
- Acrotech Biopharma
- Assertio Holdings
- Amgen
- Kyowa Hakko Kirin Co., Ltd.
- Teva B.V.
- G1 Therapeutics
- Spectrum Pharmaceuticals
- Enzychem Lifesciences Corporation
- Aileron Therapeutics
- Cellerant Therapeutics
- Myelo therapeutics
- BeyondSpring Pharmaceuticals
- Evive Biotech
- Spectrum Pharmaceuticals, and others
Chemotherapy-induced Neutropenia Market Segmentation
DelveInsight’s ‘Chemotherapy-induced Neutropenia Market Insights, Epidemiology, and Market Forecast – 2034’ report provides a detailed outlook of the current and future chemotherapy-induced neutropenia market, segmented within countries, by therapies, and by classes. Further, the market of each region is then segmented by each therapy to provide a detailed view of the current and future market share of all therapies.
Chemotherapy-induced Neutropenia Market Size by Countries
The chemotherapy-induced neutropenia market size is assessed separately for various countries, including the United States, EU4 (Germany, France, Italy, and Spain), the UK, and Japan. In 2024, the United States held a significant share of the overall 7MM (Seven Major Markets) chemotherapy-induced neutropenia market, primarily attributed to the country’s higher incidence of the condition and the elevated cost of the available treatments. This dominance is projected to persist, especially with the potential early introduction of new products.
Chemotherapy-induced Neutropenia Market Size by Therapies
Chemotherapy-induced Neutropenia Market Size by Therapies is categorized into current and emerging markets for the study period 2020–2034.
Note: Detailed market segment assessment will be provided in the final report...
Chemotherapy-induced Neutropenia Drugs Uptake
This section focuses on the sales uptake of potential chemotherapy-induced neutropenia drugs that have recently been launched or are anticipated to be launched in the chemotherapy-induced neutropenia market between 2020 and 2034. It estimates the market penetration of chemotherapy-induced neutropenia drugs for a given country, examining their impact within and across classes and segments. It also touches upon the financial and regulatory decisions contributing to the probability of success (PoS) of the drugs in the chemotherapy-induced neutropenia market. The emerging chemotherapy-induced neutropenia therapies are analyzed based on various attributes such as safety and efficacy in randomized clinical trials, order of entry and other market dynamics, and the unmet need they fulfill in the chemotherapy-induced neutropenia market.
Chemotherapy-induced Neutropenia Clinical Trials Activities
The Chemotherapy-induced Neutropenia pipeline report provides insights into Chemotherapy-induced Neutropenia Clinical Trials within Phase II, and Phase III stage. It also analyses Chemotherapy-induced Neutropenia key players involved in developing targeted therapeutics.
Chemotherapy-induced Neutropenia Pipeline Development Activities
The Chemotherapy-induced Neutropenia clinical trials analysis report covers the detailed information of collaborations, acquisition, and merger, licensing, patent details, and other information for Chemotherapy-induced Neutropenia emerging therapies.
Note: Detailed assessment of drug uptake and attribute analysis will be provided in the full report on Chemotherapy-induced Neutropenia...
Chemotherapy-induced Neutropenia Market Access and Reimbursement
DelveInsight’s ‘Chemotherapy-induced Neutropenia Market Insights, Epidemiology, and Market Forecast – 2034’ report provides a descriptive overview of the market access and reimbursement scenario of chemotherapy-induced neutropenia. This section includes a detailed analysis of the country-wise healthcare system for each therapy, enlightening the market access, reimbursement policies, and health technology assessments.
Latest KOL Views on Chemotherapy Induced Neutropenia Market Report
To keep up with current chemotherapy-induced neutropenia market trends and fill gaps in secondary findings, we interview KOLs and SMEs’ working in the chemotherapy-induced neutropenia domain. Their opinion helps understand and validate current and emerging therapies and treatment patterns or chemotherapy-induced neutropenia market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the chemotherapy-induced neutropenia unmet needs.
Chemotherapy-induced Neutropenia KOL Insights
DelveInsight’s analysts connected with 50+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. These KOLs were from organizations, institutes, and hospitals, such as University of Washington, US, University Hospital Cologne, Germany, University of Barcelona, Barcelona, Spain, Institut de Cancérologie, France, Duke University, UK, and Kobe University, Japan, among others.
“Granulocyte colony-stimulating factors (G-CSFs) work by stimulating the production and activation of neutrophils, boosting their numbers and movement in the bloodstream. They may be given as primary or secondary prevention to lower the risk, severity, and duration of febrile neutropenia, or used alongside treatment to help maintain dose-dense (more frequent) or dose-intense (higher dose) myelosuppressive chemotherapy schedules.”
“All chemotherapy patients are at risk for chemotherapy-induced neutropenia, with higher likelihood in those who are older, have poor functional or nutritional status, comorbidities, certain cancers, prior chemotherapy, advanced disease, specific regimens, or combination therapy. Diabetes or hyperglycemia increases this risk by 32%.”
“Antibiotic therapy may be continued until the absolute neutrophil count (ANC) reaches at least 500 cells/mm³ or the infection has resolved. If neutropenia persists after completing the appropriate treatment, oral fluoroquinolone prophylaxis can be resumed, provided all signs and symptoms of documented infection have resolved, and continued until bone marrow recovery occurs.”
Note: Detailed assessment of KOL Views will be provided in the full report Chemotherapy-induced Neutropenia...
Chemotherapy Induced Neutropenia Competitive Intelligence Analysis
We conduct a Competitive and Market Intelligence analysis of the chemotherapy-induced neutropenia market, utilizing various Competitive Intelligence tools such as SWOT analysis and Market entry strategies. The inclusion of these analyses is contingent upon data availability, ensuring a comprehensive and well-informed assessment of the market landscape and competitive dynamics.
Scope of the Chemotherapy-induced Neutropenia Market Report
- The Chemotherapy-induced Neutropenia treatment market report covers the descriptive overview of Chemotherapy-induced Neutropenia, explaining its causes, signs and symptoms, pathophysiology, diagnosis and currently available Chemotherapy-induced Neutropenia therapies
- Comprehensive insight has been provided into the Chemotherapy-induced Neutropenia epidemiology and treatment in the 7MM
- Additionally, an all-inclusive account of both the current and emerging therapies for Chemotherapy-induced Neutropenia is provided, along with the assessment of new therapies, which will have an impact on the current treatment landscape
- A detailed review of the Chemotherapy-induced Neutropenia treatment market; historical and forecasted is included in the report, covering drug outreach in the 7MM
- The Chemotherapy-induced Neutropenia treatment market report provides an edge while developing business strategies, by understanding trends shaping and driving the global Chemotherapy-induced Neutropenia market
Chemotherapy-induced Neutropenia Market Report Insights
- Patient-based Chemotherapy Induced Neutropenia Market Forecasting
- Chemotherapy Induced Neutropenia Therapeutic Approaches
- Chemotherapy-induced Neutropenia Pipeline Analysis
- Chemotherapy-induced Neutropenia Market Size and Trends
- Chemotherapy-induced Neutropenia Market Opportunities
- Impact of Upcoming Chemotherapy Induced Neutropenia Therapies
Chemotherapy-induced Neutropenia Market Report Key Strengths
- 10 Years Chemotherapy Induced Neutropenia Market Forecast
- The 7MM Coverage
- Chemotherapy-induced Neutropenia Epidemiology Segmentation
- Key Cross Competition
- Highly Analyzed Chemotherapy-induced Neutropenia Market
- Chemotherapy-induced Neutropenia Drugs Uptake
Chemotherapy-induced Neutropenia Market Report Assessment
- Current Chemotherapy-induced Neutropenia Treatment Practices
- Chemotherapy Induced Neutropenia Unmet Needs
- Chemotherapy-induced Neutropenia Market Attractiveness
- Chemotherapy Induced Neutropenia Market Drivers
- Chemotherapy Induced Neutropenia Market Barriers
Key Questions Answered in the Chemotherapy Induced Neutropenia Market Report:
- How common is Chemotherapy-induced Neutropenia?
- What are the key findings of Chemotherapy-induced Neutropenia epidemiology across the 7MM, and which country will have the highest number of patients during the study period (2020–2034)?
- What are the currently available treatments for Chemotherapy-induced Neutropenia?
- What are the disease risk, burden, and unmet needs of Chemotherapy-induced Neutropenia?
- At what CAGR is the Chemotherapy-induced Neutropenia market and its epidemiology is expected to grow in the 7MM during the forecast period (2025–2034)?
- How would the unmet needs impact the Chemotherapy-induced Neutropenia market dynamics and subsequently influence the analysis of the related trends?
- What would be the forecasted patient pool of Chemotherapy-induced Neutropenia in the 7MM covering the United States, EU4 (Germany, France, Italy, and Spain), the UK, and Japan?
- Among EU4 and the UK, which country will have the highest number of patients during the forecast period (2025–2034)?
- How many companies are currently developing therapies for the treatment of Chemotherapy-induced Neutropenia?
Reasons to Buy the Chemotherapy Induced Neutropenia Market Forecast Report
- The Chemotherapy Induced Neutropenia therapeutics market report will help in developing business strategies by understanding the latest trends and changing treatment dynamics driving the Chemotherapy-induced Neutropenia Drugs Market.
- Insights on patient burden/disease Chemotherapy Induced Neutropenia Incidence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- To understand the existing market opportunity in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of current treatment in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identification of strong upcoming players in the market will help in devising strategies that will help in getting ahead of competitors.
- Detailed analysis and ranking of class-wise potential current and emerging therapies under the conjoint analysis section to provide visibility around leading classes.
- Highlights of Access and Reimbursement policies of approved therapies, barriers to accessibility of off-label expensive therapies, and patient assistance programs.
- To understand the perspective of Key Opinion Leaders around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing Chemotherapy Induced Neutropenia Drugs Market so that the upcoming players can strengthen their development and launch strategy.
Stay updated with us for Recent Articles